SAB 022 2001 P31-46 Patterns of Success Among Introduced Birds In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessing the Status of Handsome Francolin Francolinus Nobilis in Bwindi Impenetrable National Park, Western Uganda
Scopus 25: 4150, December 2005 Assessing the status of Handsome Francolin Francolinus nobilis in Bwindi Impenetrable National Park, western Uganda Richard Ssemmanda and Richard A. Fuller The Handsome Francolin is a ground-dwelling partridge occurring in montane forest and the high-altitude bamboo zone within a restricted area along the Albertine Rift Mountains from the Bleus Mountains in eastern Democratic Republic of the Congo (DRC) south to Burundi (Urban et al. 1986, Madge & McGowan 2002). It appears to occur over a global range of about 120 000 km2 (Fuller et al. 2000, BirdLife International 2004), although there are very few recent records from most parts of this area. The small area of the Albertine Rift Mountains supports 41 endemic bird species, 11 of which are globally threatened (BirdLife International 2004, Plumptre et al. 2003). Forest covers much of the 56 000 km2 in the Albertine Rift Endemic Bird Area, and much of it is rugged terrain very difficult to access (Shaw & Shewry 2001). Despite this, there are significant human pressures in the area, stemming mostly from large increases in human population densities as a result of refugee movements (Omari et al. 1999, Gatarabirwa et al. 2000). Being large, slow-moving, palatable terrestrial birds, the Galliformes are under particularly direct pressure from humans through hunting and disturbance, and they may provide useful rapid indicators of the amount of human pressure in a particular area. The area surrounding Bwindi is one of Ugandas most densely populated rural areas with human densities of 160320 people/km2. Approximately 10 000 families cultivate the land immediately surrounding the park (Butynski 1984). -

A Molecular Phylogeny of the Pheasants and Partridges Suggests That These Lineages Are Not Monophyletic R
Molecular Phylogenetics and Evolution Vol. 11, No. 1, February, pp. 38–54, 1999 Article ID mpev.1998.0562, available online at http://www.idealibrary.com on A Molecular Phylogeny of the Pheasants and Partridges Suggests That These Lineages Are Not Monophyletic R. T. Kimball,* E. L. Braun,*,† P. W. Zwartjes,* T. M. Crowe,‡,§ and J. D. Ligon* *Department of Biology, University of New Mexico, Albuquerque, New Mexico 87131; †National Center for Genome Resources, 1800 Old Pecos Trail, Santa Fe, New Mexico 87505; ‡Percy FitzPatrick Institute, University of Capetown, Rondebosch, 7700, South Africa; and §Department of Ornithology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024-5192 Received October 8, 1997; revised June 2, 1998 World partridges are smaller and widely distributed in Cytochrome b and D-loop nucleotide sequences were Asia, Africa, and Europe. Most partridge species are used to study patterns of molecular evolution and monochromatic and primarily dull colored. None exhib- phylogenetic relationships between the pheasants and its the extreme or highly specialized ornamentation the partridges, which are thought to form two closely characteristic of the pheasants. related monophyletic galliform lineages. Our analyses Although the order Galliformes is well defined, taxo- used 34 complete cytochrome b and 22 partial D-loop nomic relationships are less clear within the group sequences from the hypervariable domain I of the (Verheyen, 1956), due to the low variability in anatomi- D-loop, representing 20 pheasant species (15 genera) and 12 partridge species (5 genera). We performed cal and osteological traits (Blanchard, 1857, cited in parsimony, maximum likelihood, and distance analy- Verheyen, 1956; Lowe, 1938; Delacour, 1977). -

The Northern Cardinal Margaret Murphy, Horticulture Educator and Regional Food Coordinator
Week of February 2, 2015 For Immediate Release The Northern Cardinal Margaret Murphy, Horticulture Educator and Regional Food Coordinator The northern cardinal is probably the most recognizable bird in the back yard – at least the male. He wears brilliant red plumage throughout the year. It is this amazing color that has made him the subject in many photographs and artists’ drawings, especially in winter and often on Christmas cards. Nothing is quite as cheery as seeing this holiday red bird sitting on a tree branch with a snowy scene in the background. But let’s not forget the female cardinal. While she lacks the bright red feathers, she does hold the distinction of being one of few female songbirds in North America who sing. She often does so while sitting on the nest. According to the Cornell Lab of Ornithology All About Birds website, a mated pair of northern cardinals will share song phrases. Northern cardinals are found in our region throughout the year. They are common to back yards and are frequently heard, though not always seen, perched in the higher branches of trees or shrubs while singing. They nest in shrubs, thickets or sometimes in low areas of evergreens and build their nests in the forks of small branches hidden in the dense foliage. The female builds a cup-shaped nest made of twigs. She will crush the twigs with her beak and bend them around her while she turns in the nest. She then pushes the twigs into the cup shape with her feet. The nest is lined with finer materials such as grasses or hair. -

Northern Cardinal - Cardinalis Cardinalis
Northern Cardinal - Cardinalis cardinalis Photo credit: commons.wikimedia.com. Female in left photo, male in right photo. The Northern cardinal is easily distinguishable from other birds by their bright red color and prominent crest, the Northern Cardinal is a territorial songbird often found in gardens as well as around the Garden City Bird Sanctuary. They prefer nesting in shrubs and trees. Bird Statistics Description Scientific name- Cardinalis cardinalis Size and Shape- Mid-sized songbird with a Family- Cardinalidae short and stout beak, a long tail, and a crested Conservation Status- Least concern head Length- 8.3-9.1 in (21-23 cm) Color Pattern- Males are mostly bright red Wingspan- 9.8-12.2 in (25-31 cm) with darker red wings. Females are more Weight- 1.5-1.7 oz (42-48 g) grayish tinged with red. Both sexes have a reddish beak and black around the beak Egg Statistics Behavior- Often found foraging on or near the Color- Brownish or grayish white speckled with ground near shrubbery. Males are territorial dark brown and can be aggressive Nest- Bowl-shaped that consists of twigs, leaves, Song- Series of high-pitched chirps and grasses Habitat- Live in dense and brushy areas such Clutch size- 2-5 eggs as edges of woodlands, thickets, swamps, Number of broods- 1-2 overgrown fields, and gardens Length- 0.9-1.1 in (1.7-2 cm) Range- Year-round in northern and central Width- 0.7-0.8 in (1.7-2 cm) part of the US, parts of southern Canada, Incubation period- 11-13 days Mexico, and around the Gulf of Mexico Nestling period- 7-13 days Diet- Mostly grains, seeds, and fruits from bird feeders and the ground supplemented with Additional Information: insects such as grasshoppers and cicadas http://en.wikipedia.org/wiki/Northern_cardinal http://birds.audubon.org/birds/northern- cardinal Thanks to Crystal Chang for this bird data page. -
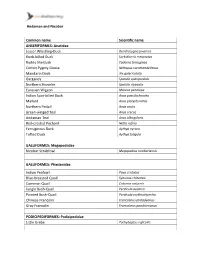
Andaman and Nicobar Common Name Scientific Name
Andaman and Nicobar Common name Scientific name ANSERIFORMES: Anatidae Lesser Whistling-Duck Dendrocygna javanica Knob-billed Duck Sarkidiornis melanotos Ruddy Shelduck Tadorna ferruginea Cotton Pygmy-Goose Nettapus coromandelianus Mandarin Duck Aix galericulata Garganey Spatula querquedula Northern Shoveler Spatula clypeata Eurasian Wigeon Mareca penelope Indian Spot-billed Duck Anas poecilorhyncha Mallard Anas platyrhynchos Northern Pintail Anas acuta Green-winged Teal Anas crecca Andaman Teal Anas albogularis Red-crested Pochard Netta rufina Ferruginous Duck Aythya nyroca Tufted Duck Aythya fuligula GALLIFORMES: Megapodiidae Nicobar Scrubfowl Megapodius nicobariensis GALLIFORMES: Phasianidae Indian Peafowl Pavo cristatus Blue-breasted Quail Synoicus chinensis Common Quail Coturnix coturnix Jungle Bush-Quail Perdicula asiatica Painted Bush-Quail Perdicula erythrorhyncha Chinese Francolin Francolinus pintadeanus Gray Francolin Francolinus pondicerianus PODICIPEDIFORMES: Podicipedidae Little Grebe Tachybaptus ruficollis Andaman and Nicobar COLUMBIFORMES: Columbidae Rock Pigeon Columba livia Andaman Wood-Pigeon Columba palumboides Eurasian Collared-Dove Streptopelia decaocto Red Collared-Dove Streptopelia tranquebarica Spotted Dove Streptopelia chinensis Laughing Dove Streptopelia senegalensis Andaman Cuckoo-Dove Macropygia rufipennis Asian Emerald Dove Chalcophaps indica Nicobar Pigeon Caloenas nicobarica Andaman Green-Pigeon Treron chloropterus Green Imperial-Pigeon Ducula aenea Nicobar Imperial-Pigeon Ducula nicobarica Pied Imperial-Pigeon -

2020 Dodgy Drongo Twitchathon Report the Drongos Last Won the 30Hr Race Back in 2017 and It Was Great Getting That Team Back Together Again
2020 Dodgy Drongo Twitchathon Report The Drongos last won the 30hr race back in 2017 and it was great getting that team back together again. Maxie the Co-pilot and Simon ‘The Whisperer’ joined the Capt’n for a week of birding out west before heading to our starting point in the mulga. It is here I should mention that the area is currently seeing an absolute boom period after recent rains and was heaving with birds. So with this in mind and with our proven ‘golden route’ we were quite confident of scoring a reasonably good score. We awoke to rain on tin and it didn’t stop as we drove to the mulga, in fact it got heavier.As we sat in the car with 20min until start time we were contemplating if this would kill our run. But as we walked around in the drizzle we soon realised the birds were still active. Simon found a family of Inland Thornbill (a bird I always like to start with), Max had found a roosting Hobby and I’d found some Bee-eaters and Splendid Fairywrens. As the alarm chimed we quickly ticked up all those species as well as Red-capped Robin, Masked and White-browed Woodswallow, Black Honeyeater, White-winged Triller and Budgerigar. The Little Eagle was still on it’s nest and a pair of Plum-headed Finches shot past. We then changed location within the mulga and soon flushed a Little Buttonquail!! Common Bronewing, Owlet-nightjar and Mulga Parrot soon fell and after 20min we were off. -

Peninsular Malaysia 2018
Report of a Birding Trip to Peninsular Malaysia From 25th February to 4th March 2018 Participants: Arjan Brenkman & Jan van der Laan Malayan Partridge, 26 February 2018, Fraser’s Hill, Malaysia; © Jan van der Laan. Birds observed on the Peninsular Malaysia between 25 February and 4 March 2018 Map of Peninsular Malaysia; © Google Maps. Map Fraser’s Hill; © https://www.journeymalaysia.com/MH_fraser.htm © The Virtual Birders 2018 2 Birds observed on the Peninsular Malaysia between 25 February and 4 March 2018 Map of Taman Negara; © Park HQ. River trail and bridge towards Tabing Hide, Taman Negara; © Arjan Brenkman. © The Virtual Birders 2018 3 Birds observed on the Peninsular Malaysia between 25 February and 4 March 2018 Navigation error: the latitude was correct, the longitude was incorrectly put in the navigation system. This is what it should have been. We found out this error just an hour we arrived before Sungai Koyan; it took us 1:45 minutes extra. _____________ © The Virtual Birders 2018 4 Birds observed on the Peninsular Malaysia between 25 February and 4 March 2018 Introduction It was only ten months ago since our successful trip to Taiwan in 2017 and this time we decided to go to Peninsular Malaysia to see Garnet and Rusty-naped Pitta plus the Mountain Peacock-Pheasant as quickly as possible. It was also a good opportunity to look for some missing Sundae species. We would focus on three areas, the Genting Highlands (for the Mountain Peacock-Pheasant), Fraser’s Hill (Malayan Partridge, Malayan Whistling- Thrush, Black-Laughingthrush and Rusty-naped Pitta) and Taman Negara (lowland species like Garnet Pitta, Malayan Banded Pitta, Short-toed Coucal, Black-throated Babbler, White-necked Babbler, Large Wren-Babbler and Rail-Babbler). -

Ammoperdix Griseogularis, Galliformes) Populations from Sub-Himalayan Mountain Ranges of Pakistan
Belg. J. Zool., 140 (2) : 229-234 July 2010 Genetic diversity in see-see partridge (Ammoperdix griseogularis, Galliformes) populations from sub-Himalayan Mountain ranges of Pakistan Imran Khaliq1,3*, Muhammad Babar2, Maria Riaz1 & Aleem Ahmed Khan1 1 Institute of Pure & Applied Biology, Bahauddin Zakariya University, Multan 60800, Pakistan 2 Institute of Biotechnology, Bahauddin Zakariya University, Multan 60800, Pakistan 3 Government College Dera Ghazi Khan, 32200, Pakistan * Corresponding author: [email protected] ABSTRACT. We used Random Amplified Polymorphic DNA (RAPD) markers to investigate the genetic structure of two popula- tions of see-see partridge (Ammoperdix griseogularis, Galliformes) from the Suleiman range, in the Pakistani Himalayan region. The see-see partridge is a vulnerable species with a distribution in the Middle East and central Asia. The percentage of polymorphic bands (94.05%), Shannon Index (H=0.455) and Nei’s average gene diversity (IN=0.298) of A. griseogularis at species level were rather high when compared with other avian species. 17% of polymorphic loci showed statistically significant differences in their allelic frequencies. The GST (Nei’s coefficient of genetic variation) values indicated low levels of differentiation (GST=0.08). A genetic distance D of 0.05 indicated that both populations were to some degree in isolation but their differentiation was not signifi- cant. Overall, our genetic data can support action plans aiming to locally preserve differentiated genetic resources that, in the future, could potentially result in ecologically and behaviourally differentiated populations. In view of the rapid environmental changes that the Himalayan region has been experiencing in the last decade, this study could help in conservation plans. -

(2004): Identification, Distribution, and Function of Gastroliths in Dinosaurs
IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Diplodocid sauropod accidentally ingesting gastroliths while feeding on a cycad. IDENTIFICATION, DISTRIBUTION, AND FUNCTION OF GASTROLITHS IN DINOSAURS AND EXTANT BIRDS WITH EMPHASIS ON OSTRICHES (STRUTHIO CAMELUS) Dissertation zur Erlangung des Doktorgrades (Dr. rer. nat.) der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn vorgelegt von Oliver Wings aus Sangerhausen Bonn 2004 Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn 1. Referent: Privat-Dozent Dr. Martin Sander 2. Referent: Professor Dr. Jes Rust Tag der Promotion: 02.12.2004 Diese Dissertation ist auf dem Hochschulschriftenserver der ULB Bonn http://hss.ulb.uni- bonn.de/diss_online elektronisch publiziert This dissertation is published electronically on the ULB Bonn server for university publications: http://hss.ulb.uni-bonn.de/diss_online Dedicated to Claudia. Thanks for everything. French proverb: “Il a un estomac d’autuche!” (literally: He has the stomach of an ostrich!) means: He can tolerate everything! Geheimnisvoll am lichten Tag Läßt sich Natur des Schleiers nicht berauben, Und was sie deinem Geist -

Malaysia & Borneo Trip Report
Malaysia & Borneo Trip Report Rainforest Birds & Mammals th th 8 to 26 June 2015 The scarce and beautiful Jambu Fruit Dove, Taman Nagara by Rosemary Loyd RBT Trip Report Malaysia & Borneo 2015 2 The rare Whitehead’s Trogon, Mt Kinabalu and a male Crested Fireback, Taman Nagara, both by Butch Carter Top Ten Birds as voted by the participants: 1) Whitehead’s Broadbill 2) Whitehead’s Trogon 3) Jambu Fruit Dove 4) Bornean Green Magpie 5) Long-tailed Broadbill 6) Buffy Fish Owl 7) Oriental Dwarf Kingfisher/Hooded Pitta 8) Temminck’s Sunbird 9) Rhinoceros Hornbill 10) Crested Fireback Mammals: 1) Malaysian Tapir 2) Orangutan 3) Proboscis Monkey 4) Small-clawed Otter RBT Trip Report Malaysia & Borneo 2015 3 Tour Leaders: Dennis Yong and Erik Forsyth Tour report compiled by Tour Leader: Erik Forsyth Temminck’s Sunbird by John Clark Tour Summary On this year’s tour we recorded the rare and highly prized Whitehead’s Trogon, Whitehead’s Broadbill (after a long search on Mount Kinabalu) and Garnet, Blue-headed, Black-crowned and Hooded Pittas. Other mouthwatering species seen were Rhinoceros, Wreathed, Wrinkled and Black Hornbills, White- fronted and Black-thighed Falconets, Black-and-red, Black-and-yellow, Long-tailed, Dusky, Green and Banded Broadbills, the stunning Oriental Dwarf, Blue-eared, Blue-banded and Stork-billed Kingfishers, Red-headed, Diard’s, Red-naped and Scarlet-rumped Trogons, Great-billed Heron, Painted and Storm’s Storks, Lesser Adjutant, Wallace’s, Rufous-bellied and Blyth’s Hawk-Eagles, Crested Fireback, Buffy Fish and Brown Wood Owls, the highly sought-after Bornean Bristlehead and Blue Nuthatch, the endangered Straw-headed Bulbul, a whopping eight sightings of Orangutan and several troops of Proboscis Monkey, Malaysian Tapir and Western Tarsier. -

Brookfield CP Bird List
Bird list for BROOKFIELD CONSERVATION PARK -34.34837 °N 139.50173 °E 34°20’54” S 139°30’06” E 54 362200 6198200 or new birdssa.asn.au ……………. …………….. …………… …………….. … …......... ……… Observers: ………………………………………………………………….. Phone: (H) ……………………………… (M) ………………………………… ..………………………………………………………………………………. Email: …………..…………………………………………………… Date: ……..…………………………. Start Time: ……………………… End Time: ……………………… Codes (leave blank for Present) D = Dead H = Heard O = Overhead B = Breeding B1 = Mating B2 = Nest Building B3 = Nest with eggs B4 = Nest with chicks B5 = Dependent fledglings B6 = Bird on nest NON-PASSERINES S S A W Code No. NON-PASSERINES S S A W Code No. NON-PASSERINES S S A W Code No. Rainbow Bee-eater Mulga Parrot Eastern Bluebonnet Red-rumped Parrot Australian Boobook *Feral Pigeon Common Bronzewing Crested Pigeon Australian Bustard Spur-winged Plover Little Buttonquail (Masked Lapwing) Painted Buttonquail Stubble Quail Cockatiel Mallee Ringneck Sulphur-crested Cockatoo (Australian Ringneck) Little Corella Yellow Rosella Black-eared Cuckoo (Crimson Rosella) Fan-tailed Cuckoo Collared Sparrowhawk Horsfield's Bronze Cuckoo Grey Teal Pallid Cuckoo Shining Bronze Cuckoo Peaceful Dove Maned Duck Pacific Black Duck Little Eagle Wedge-tailed Eagle Emu Brown Falcon Peregrine Falcon Tawny Frogmouth Galah Brown Goshawk Australasian Grebe Spotted Harrier White-faced Heron Australian Hobby Nankeen Kestrel Red-backed Kingfisher Black Kite Black-shouldered Kite Whistling Kite Laughing Kookaburra Banded Lapwing Musk Lorikeet Purple-crowned Lorikeet Malleefowl Spotted Nightjar Australian Owlet-nightjar Australian Owlet-nightjar Blue-winged Parrot Elegant Parrot If Species in BOLD are seen a “Rare Bird Record Report” should be submitted. SEASONS – Spring: September, October, November; Summer: December, January, February; Autumn: March, April May; Winter: June, July, August IT IS IMPORTANT THAT ONLY BIRDS SEEN WITHIN THE RESERVE ARE RECORDED ON THIS LIST. -

Demonstration of the Potential of Environmental DNA As A
www.nature.com/scientificreports OPEN Demonstration of the potential of environmental DNA as a tool for the detection of avian species Received: 16 October 2017 Masayuki Ushio 1,2, Koichi Murata3,4, Tetsuya Sado5, Isao Nishiumi6, Masamichi Takeshita7, Accepted: 1 March 2018 Wataru Iwasaki 7 & Masaki Miya 5 Published: xx xx xxxx Birds play unique functional roles in the maintenance of ecosystems, such as pollination and seed dispersal, and thus monitoring bird species diversity is a frst step towards avoiding undesirable consequences of anthropogenic impacts on bird communities. In the present study, we hypothesized that birds, regardless of their main habitats, must have frequent contact with water and that tissues that contain their DNA that persists in the environment (environmental DNA; eDNA) could be used to detect the presence of avian species. To this end, we applied a set of universal PCR primers (MiBird, a modifed version of fsh/mammal universal primers) for metabarcoding avian eDNA. We confrmed the versatility of MiBird primers by performing in silico analyses and by amplifying DNAs extracted from bird tissues. Analyses of water samples from zoo cages of birds with known species composition suggested that the use of MiBird primers combined with Illumina MiSeq could successfully detect avian species from water samples. Additionally, analysis of water samples collected from a natural pond detected fve avian species common to the sampling areas. The present fndings suggest that avian eDNA metabarcoding would be a complementary detection/identifcation tool in cases where visual census of bird species is difcult. Environmental DNA (eDNA) is genetic material that persists in an environment and is derived from organ- isms living there, and researchers have recently been using eDNA to detect the presence of macro-organisms, particularly those living in aquatic/semiaquatic ecosystems1–5.