Dicyclopentadiene Hydroformylation to Value- Added Fine Chemicals Over Magnetically Separable Fe3o4-Supported Co-Rh Bimetallic Catalysts: Effects of Cobalt Loading
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Air Contaminants – Permissible Exposure Limits (Pels)
SUBPART Z -- TOXIC AND HAZARDOUS SUBSTANCES 1910.1000-AIR CONTAMINANTS An employee’s exposure to any substance listed in Table Z-1-A of this section shall be limited in accordance with the requirements of the following paragraphs of this section. (a) Table Z-1-A. Limits for Air Contaminants (1) & (2) Enforcement of Transitional Limits has expired. See Paragraph (3) for Limits. (3) Limits for Air Contaminants Columns. An employee’s exposure to any substance listed in Table Z-1-A shall not exceed the Time Weighted Average (TWA), Short Term Exposure Limit (STEL) and Ceiling Limit specified for that substance in Table Z-1-A. (4) Skin Designation. To prevent or reduce skin absorption, an employee’s skin exposure to substances listed in Table Z-1-A with an “X” in the Skin Designation column following the substance name shall be prevented or reduced to the extent necessary in the circumstances through the use of gloves, coveralls, goggles, or other appropriate personal protective equipment, engineering controls or work practices. (5) Definitions. The following definitions are applicable to the Limits for Air Contaminants columns of Table Z- 1-A: (i) Time weighted average (TWA) is the employee’s average airborne exposure in any 8-hour work shift of a 40-hour work week which shall not be exceeded. (ii) Short term exposure limit (STEL) is the employee’s 15-minute time weighted average exposure which shall not be exceeded at any time during a work day unless another time limit is specified in a parenthetical notation below the limit. -

The Synthetization and Analysis of Dicyclopentadiene and Ethylidene-Norbornene Microcapsule Systems
polymers Article The Synthetization and Analysis of Dicyclopentadiene and Ethylidene-Norbornene Microcapsule Systems Ionut Sebastian Vintila 1,2,*, Horia Iovu 2, Andreea Alcea 1, Andreia Cucuruz 3, Andrei Cristian Mandoc 1 and Bogdan Stefan Vasile 4 1 National Research and Development Institute for Gas Turbines COMOTI, 061126 Bucharest, Romania; [email protected] (A.A.); [email protected] (A.C.M.) 2 Department of Bioresources and Polymer Science, Faculty of Applied Chemistry and Materials Science, University Politehnica of Bucharest, 011061 Bucharest, Romania; [email protected] 3 Department of Science and Engineering of Oxide Materials and Nanomaterials, Faculty of Applied Chemistry and Materials Science, University Politehnica of Bucharest, 011061 Bucharest, Romania; [email protected] 4 National Research Centre for Micro and Nanomaterials, Faculty of Applied Chemistry and Materials Science, University POLITEHNICA of Bucharest, 060042 Bucharest, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-726998218 Received: 7 April 2020; Accepted: 25 April 2020; Published: 4 May 2020 Abstract: The activities of this paper were focused on an in-situ fabrication process for producing two self-healing systems containing dicyclopentadiene and 5-ethylidene-2-norbornene monomers encapsulated in a urea-formaldehyde shell and integration methods applied in the epoxy matrix to analyse and compare the influences of their integration into the neat epoxy matrix. The self-healing systems were first synthesized according to a literature review, and subsequently, an optimization process was conducted for the fabrication process. Neat epoxy specimens were fabricated as reference specimens and subjected to flexural tests. Several integration methods for incorporating the self-healing systems into the epoxy resin were investigated. -

Experiment #4 the Preparation of Ferrocene & Acetylferrocenes1
Experiment #4: The Preparation of Ferrocene & Acetylferrocene MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Chemistry 5.311 Introductory Chemical Experimentation EXPERIMENT #4 THE PREPARATION OF FERROCENE & ACETYLFERROCENES1 I. Purpose The principal aims of this experiment are to provide background information about and experience in: S the synthesis and electronic structure of ferrocene (1), ( -C5H5)2Fe [bis(pentahaptocyclopentadienyl) iron]2 S the synthesis of an ionic liquid, as environment for the acetylation of ferrocene S the use of inert atmospheres (including glove box techniques) S recrystallization or sublimation S the use of GC/MS and thin-layer chromatography as analytical tools and column chromatography as a purification technique • the concepts of Green Chemistry such as Atom Economy3 and alternative non-polluting solvents Ferrocene is a historically important molecule. The initial recognition of the structure of 4 C10H10Fe in 1951 spawned a vast area of chemistry, viz., transition metal organometallic chemistry. This field is still developing and has produced a huge number of compounds in which saturated, unsaturated, and aromatic organic fragments are bonded directly to metals. All carbon atoms in the two cyclopentadienyl rings are equally bonded to the central ion by electrons in the rings. The sandwich structure proposed by Wilkinson and Fischer5,6 (Nobel prize in 1973) in early 1 Mircea D. Gheorghiu designed the experiment to include the acetylation of ferrocene in ionic liquid. 2 The word hapto means in Greek to fasten. Pentahapto therefore, is to be taken as “fastened in five places.” The greek letter followed by a superscript indicates how many atoms of the ligand are attached to the metal. -

Peroxides and Peroxide- Forming Compounds
FEATURE Peroxides and peroxide- forming compounds By Donald E. Clark Bretherick5 included a discussion of nated. However, concentrated hydro- organic peroxide5 in a chapter on gen peroxide (Ͼ30%), in contact with norganic and organic peroxides, highly reactive and unstable com- ordinary combustible materials (e.g., because of their exceptional reac- pounds and used “oxygen balance” to fabric, oil, wood, or some resins) Itivity and oxidative potential are predict the stability of individual com- poses significant fire or explosion haz- widely used in research laboratories. pounds and to assess the hazard po- ards. Peroxides of alkali metals are not This review is intended to serve as a tential of an oxidative reaction. Jack- particularly shock sensitive, but can 6 guide to the hazards and safety issues son et al. addressed the use of decompose slowly in the presence of associated with the laboratory use, peroxidizable chemicals in the re- moisture and may react violently with handling, and storage of inorganic and search laboratory and published rec- a variety of substances, including wa- organic peroxy-compounds and per- ommendations for maximum storage ter. Thus, the standard iodide test for oxide-forming compounds. time for common peroxide-forming peroxides must not be used with these The relatively weak oxygen-oxygen laboratory solvents. Several solvents, water-reactive compounds.1 linkage (bond-dissociation energy of (e.g., diethyl ether) commonly used in Inorganic peroxides are used as ox- 20 to 50 kcal moleϪ1) is the character- the laboratory can form explosive re- idizing agents for digestion of organic istic structure of organic and inor- action products through a relatively samples and in the synthesis of or- ganic peroxide molecules, and is the slow oxidation process in the pres- ganic peroxides. -

Synthesis and Reactivity of Cyclopentadienyl Based Organometallic Compounds and Their Electrochemical and Biological Properties
Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Sasmita Mishra Department of Chemistry National Institute of Technology Rourkela Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Dissertation submitted to the National Institute of Technology Rourkela In partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry by Sasmita Mishra (Roll Number: 511CY604) Under the supervision of Prof. Saurav Chatterjee February, 2017 Department of Chemistry National Institute of Technology Rourkela Department of Chemistry National Institute of Technology Rourkela Certificate of Examination Roll Number: 511CY604 Name: Sasmita Mishra Title of Dissertation: ''Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties We the below signed, after checking the dissertation mentioned above and the official record book(s) of the student, hereby state our approval of the dissertation submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry at National Institute of Technology Rourkela. We are satisfied with the volume, quality, correctness, and originality of the work. --------------------------- Prof. Saurav Chatterjee Principal Supervisor --------------------------- --------------------------- Prof. A. Sahoo. Prof. G. Hota Member (DSC) Member (DSC) --------------------------- -

Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) by Pd Complexes Containing Β-Ketoiminate Or Β-Diketiminate Ligands
Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) Bull. Korean Chem. Soc. 2012, Vol. 33, No. 12 4131 http://dx.doi.org/10.5012/bkcs.2012.33.12.4131 Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) by Pd Complexes Containing β-Ketoiminate or β-Diketiminate Ligands Eung Jun Lee, Ho Sup Kim, Byoung Ki Lee, Woon Sung Hwang,† Ik Kyoung Sung,† and Ik Mo Lee* Department of Chemistry, Inha University, Incheon 402-751, Korea. *E-mail: [email protected] †R&D Center, Kolon Industries, Inc., Incheon, Korea Received August 31, 2012, Accepted September 27, 2012 Several palladium complexes containing β-ketoiminate and β-diketiminate ligands successfully produced poly(DCPD) possibly via vinyl addition. It was found that catalysts with β-diketiminate ligands containing bulkier aryl substituents showed the highest activity in the presence of MAO as a cocatalyst. Purity of DCPD is quite essential for the higher activity and small amount of organic solvent such as CH2Cl2 and toluene is required to reduce the viscosity of the reactant mixture for the higher activity. 1H NMR spectra of produced polymers with N,N-dimethylanilinium tetra(pentafluorophenyl)borate (N,N-DAPFAr”4) show that 5,6-double bond of DCPD is removed with 2,3-double bond remaining. Produced poly(DCPD) with MAO cocatalyst is quite rigid and insoluble in common organic solvents but rather brittle. Key Words : Palladium complexes, β-Ketoiminate, β-Diketiminate, Vinyl addition polymerization, Poly(DCPD) Introduction Figure 1). Therefore, vinyl addition polymerization using all the double bonds in the DCPD can proceed with an ap- Poly(DCPD) is a crosslinked thermosetting polymer with propriate catalyst, if any. -

Relative Chalcogen Donor Properties in Transition Metal Complexes. Eugene D
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1971 Relative Chalcogen Donor Properties in Transition Metal Complexes. Eugene D. Schermer Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Schermer, Eugene D., "Relative Chalcogen Donor Properties in Transition Metal Complexes." (1971). LSU Historical Dissertations and Theses. 1949. https://digitalcommons.lsu.edu/gradschool_disstheses/1949 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. 71-20,621 SCHERMER, Eugene D., 1934- RELATIVE CHALCOGEN DONOR PROPERTIES IN TRANSITION METAL COMPLEXES. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1971 Chemistry, inorganic University Microfilms, A XEROXCompany, Ann Arbor, Michigan THIS DISSERTATION HAS BEEN MICROFILMED EXACTLY AS RECEIVED Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. RELATIVE CHALCOGEN DONOR PROPERTIES IN TRANSITION METAL COMPLEXES A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Eugene D. Schemer B.A., Eastern Washington State College, 1958 M.S., Oregon State University, 1Q62 January, 197! Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGEMENT The author wishes to express his sincere gratitude to Professor William H. Baddley, under whose direction this work was produced. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -
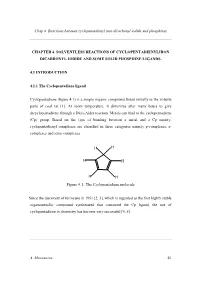
Chap4 Cpfe CO 2I + PR3 Corr
Chap 4 Reactions between cyclopentadienyl iron dicarbonyl iodide and phosphines CHAPTER 4. SOLVENTLESS REACTIONS OF CYCLOPENTADIENYLIRON DICARBONYL IODIDE AND SOME SOLID PHOSPHINE LIGANDS. 4.1 INTRODUCTION 4.1.1 The Cyclopentadiene ligand Cyclopentadiene (figure 4.1) is a simple organic compound found initially in the volatile parts of coal tar [1]. At room temperature, it dimerizes after many hours to give dicyclopentadiene through a Diels-Alder reaction. Metals can bind to the cyclopentadiene (Cp) group. Based on the type of bonding between a metal and a Cp moiety, cyclopentadienyl complexes are classified in three categories namely p-complexes, s- complexes and ionic complexes. H H H H H H Figure 4. 1. The Cyclopentadiene molecule Since the discovery of ferrocene in 1951 [2, 3], which is regarded as the first highly stable organometallic compound synthesized that contained the Cp ligand, the use of cyclopentadiene in chemistry has become very successful [4, 5]. A. Munyaneza 50 Chap 4 Reactions between cyclopentadienyl iron dicarbonyl iodide and phosphines The popularity of the use of the Cp ligand in chemistry is due to some interesting features associated with this ligand: - The Cp ligand can bind to the metal through one to five carbon atoms ( η1- η5) [6]; - The Cp ligand can stabilize both low and high oxidation state metals [7]; - By changing the functionality (all H atoms can be replaced) on the Cp ligands, the resulting compounds possess different physical and chemical properties from the normal Cp such as stability, solubility, electron donor character etc [8]. The cyclopentadiene ligand reacts with transitional metals to form cyclopentadienyl metal complexes. -

1,3-Cyclopentadiene 2523
1,3-CYCLOPENTADIENE 2523 C5H6 MW: 66.10 CAS: 542-92-7 RTECS: GY1000000 METHOD: 2523, Issue 2 EVALUATION: FULL Issue 1: 15 May 1985 Issue 2: 15 August 1994 OSHA : 75 ppm PROPERTIES: liquid; d 0.8021 g/mL @ 20 °C; NIOSH: 75 ppm BP 42 °C; MP •85 °C; dimerizes to solid ACGIH: TWA 75 ppm (MP 32.5 °C); VP not available (1 ppm = 2.70 mg/m 3 @ NTP) SYNONYMS: none SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBE TECHNIQUE: GAS CHROMATOGRAPHY, FID (maleic anhydride on Chromosorb 104, 100 mg/50 mg) ANALYTE: 1,3-cyclopentadiene-maleic anhydride adduct (see REAGENTS, 1.) FLOW RATE: 0.01 to 0.05 L/min DISSOLUTION: 10 mL ethyl acetate; stand 15 min VOL-MIN: 1 L @ 75 ppm -MAX: 5 L INJECTION VOLUME: 5 µL SHIPMENT: routine TEMPERATURE-INJECTION: 200 °C -DETECTOR: 250 °C SAMPLE -COLUMN: 155 °C STABILITY: at least 1 week @ 25 °C [1] CARRIER GAS: N2, 30 mL/min BLANKS: 2 to 10 field blanks per set COLUMN: 3 m x 3-mm OD stainless steel packed with 5% OV-17 on 100/120 mesh Chromosorb WHP ACCURACY CALIBRATION: analyte in ethyl acetate RANGE STUDIED: 73 to 370 mg/m 3 [1] (3-L samples) RANGE: 0.2 to 1.2 mg 1,3-cyclopentadiene per sample BIAS: 3.6% ˆ OVERALL PRECISION (S rT): 0.066 [2] ESTIMATED LOD: 0.01 mg per sample [1] ACCURACY: ± 16.5% PRECISION (S r): 0.031 @ 0.3 to 1.2 mg per sample [1] APPLICABILITY: The working range is 25 to 150 ppm (67 to 400 mg/m 3) for a 3-L air sample, based on sampler capacity at high relative humidity. -

UNITED STATES PATENT OFFICE 2,688,627 DCYCLOPENTADENE CARBOXYFLIC ACDS Charles A
Patented Sept. 7, 1954 2,688,627 UNITED STATES PATENT OFFICE 2,688,627 DCYCLOPENTADENE CARBOXYFLIC ACDS Charles A. Cohen, Roselle Park, Louis A. Mikeska, Westfield, and Frederick Knoth, Jr., Sayreville, N. J., assignors to Standard Oil Development Company, a corporation of Delaware No Drawing. Application May 8, 1952, Serial No. 286,834 13 Claims. (C. 260-429) 1. 2 This invention relates to novel dicyclopenta- diene is used as the starting material. In the diene carboxylic acids and to a process for pro- case of pure dicyclopentadiene the hydrogenation ducing the same. Specifically, this invention re- product is 9,10-dihydrodicyclopentadiene boiling lates to tetrahydrodicyclopentadiene carboxylic at 177° to 178° C., at atmospheric pressure and acids and process for producing these products. 5 inelts at 50 to 50.5° C. On titration with bromine In the practice of this invention the starting it absorbs bromine in an amount equal to 119 material is Substantially pure dicyclopentadiene centigrams of bromine per gram. The mixed Or a copolymer of methyl cyclopentadiene and products of hydrogenation are liquid at Ordinary cyclopentadiene (hereinafter referred to as temperature due to the mutual depression of the methyldicyclopentadiene) or a dimer made from 10 melting point. methyl cyclopentadiene (hereinafter referred to For purposes of purification and elimination of as dimethyldicyclopentadiene) or mixtures of two any unhydrogenated dimers present in the crude or more of these products normally found in dihydro product it is desirable but not necessary petroleum refinery streams originating from to distill the crude hydrogenation product at at either vapor phase, gas phase, or steam cracking 15 mospheric or higher preSSure So as to crack any processes. -

Studies of Reactions of Some Carbonyl Bridged
STUDIES OF REACTIONS OF SOME CARBONYL BRIDGED COMPOUNDS WITH IRON PENTACARBONYL LAI Chi-hung ( 黎 志 雄 ) A thesis submitted in partial fulfillment of the requirements for the degree of Master of Philosophy at The Chinese University of Hong Kong 1978 Thesis Committee: Dr. S. W. Tam, Chairman Dr. K. H. Wong Dr. T. Y. Luh Prof. A. J. Birch, F. R. S., External Examiner ACKNOWLEDGEMENT The author wishes to express his gratitude to his supervisor, Dr. S. W. Tam, for his invaluable instruction and continous encouragement throughout the course of this rsearch and the perparation of this thesis. He is indebted to Dr. T. Y. Luh, Dr. T. L. Chan, and Mr. T. C. Chiang for their helpful discussions during this investigation. His sincere thanks are also due to Mr. Y. H. Law and Mr. S. C. Wong for their assistance in the measurements of nuclear magnetic resonance spectra and mass spectra, respectively. LAI Chi-hung Department of Chemistry The Chinese University of Hong Kong May, 1978 11 SUMMARY The reactions of iron pentacarbonyl with a series of carbonyl bridged compounds, viz, 3a,4,,7,7a-tetrahydro-4,7-. methanoindene-l,,8-dione (6),.2, L4-dibromo-3a,,4,7, 7a-tetra- hydro-4,,7-methanoindene-l,,8-dione (32), 4,5, 6, 7-tetraphenyl- 3a, 4, 7, 7a-tetrahydro-4,,7-methanoindene-8-one (33) and octa- chloro-3a,4,.7,7a-tetrahydro-4,7-methanoindene-1,8-dione (39) have been investigated Treatment of (6) with iron penta- carbonyl afforded l-indanone. By using deuteriated compound (21), the course of reaction was shown first to undergo decarbonylation of the initial compound giving rise to 3a,7a- dihydroindenone (8) which was then catalyzed by iron penta- carbonyl,to undergo 1,3-hydrogen shift to yield l-indanone.