Rebateable Manufacturers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Snippet Roundup: CETP Kaput and Biogen Buys Into Amyloid
October 27, 2017 Snippet roundup: CETP kaput and Biogen buys into amyloid Edwin Elmhirst Welcome to your weekly roundup of EP Vantage’s snippets – short takes on smaller news items. This week, October 23 to 27, 2017, we had thoughts on the following: Amgen puts another CETP out of its misery; Stryker buys Vexim without waiting for US approval; How much is aducanumab worth? At least $8bn, Biogen reckons; Alcon delay puts dampener on Novartis; Soliris sitting pretty with complementary new indication; Smith & Nephew acquires, but divestments could follow; Global Blood drops IPF programme. These snippets were previously published daily via twitter. Amgen puts another CETP out of its misery October 26, 2017 If the once-promising CETP drug class lives on, it might only be in a rather restricted group of heart disease patients with a very specific mutation. Amgen became the last big biopharma group to cancel work on a drug in the HDL-raising class when it announced plans to seek a partner for AMG 899, following the lead of Merck & Co, which recently terminated its work with anacetrapib after disappointing results in the Reveal trial. Although big pharma groups obviously believe that it is unlikely for a CETP inhibitor to succeed commercially, it is not out of the realm of possibility that a partner could emerge for AMG 899 – Roche licensed its project, dalcetrapib, to the Canadian group Dalcor Pharmaceuticals, which has been researching its use in a genetic subtype, patients with an AA polymorphism at the rs1967309 location in the ADCY9 gene. Amgen will write off the $300m it paid for AMG 899’s originator, Dezima Pharma, in 2015, which at the time was seen as a cheap bet on the CETP sector. -

Abbvie Allergan Acquisition
Creating a New Diversified Biopharmaceutical Company The Combination of AbbVie and Allergan Investor Presentation June 25, 2019 NO OFFER OR SOLICITATION This presentation is not intended to and does not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities or the solicitation of any vote or approval in any jurisdiction pursuant to the acquisition or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. In particular, this presentation is not an offer of securities for sale into the United States. No offer of securities shall be made in the United States absent registration under the U.S. Securities Act of 1933, as amended, or pursuant to an exemption from, or in a transaction not subject to, such registration requirements. Any securities issued in the acquisition are anticipated to be issued in reliance upon available exemptions from such registration requirements pursuant to Section 3(a)(10) of the U.S. Securities Act of 1933, as amended. The acquisition will be made solely by means of the Scheme Document (or, if applicable, the Takeover Offer document), which will contain the full terms and conditions of the acquisition, including details with respect to the AbbVie shareholder vote in respect of the acquisition. Any decision in respect of, or other response to, the acquisition, should be made only on the basis of the information contained in the Scheme Document. IMPORTANT ADDITIONAL INFORMATION WILL BE FILED WITH THE SEC In connection with the proposed Acquisition, Allergan will file with the Securities Exchange Commission (the “SEC”) a Proxy Statement, which will include the Scheme Document. -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

2012 Financial Markets Preview Living Creatively
REPRINT FROM JANUARY 2, 2012 BioCentury ™ THE BERNSTEIN REPORT ON BIOBUSINESS Article Reprint • Page 1 of 8 2012 Financial Markets Preview Living creatively By Stacy Lawrence “As the capital markets Viren Mehta of Mehta Partners. “Many Senior Writer companies don’t have any option but to For small, early stage biotech compa- continue to be difficult, raise at lower valuations.” nies, 2012 could be the year of living traditional investors are able He added: “Many companies are not creatively. able to defer any longer. The moment Although public biotechs raised more to extract terms that are comes for many companies when they funds last year than ever before, the vast have to swallow hard and accept painful majority of the money was debt financing more and more onerous.” dilution.” by established companies with marketed products. Thus while the overall numbers Todd Wyche, Brinson Patrick look good and are likely to continue to do Cash on hand so, precommercial companies will have to large and mid-cap companies (see “The Market volatility and macroeconomic get creative with their financings. 1% Effect,” page 2). risk had most buysiders sitting on the Many of these companies avoided rais- In 2011, public biotechs raised $43 sidelines through the back half of 2011. ing money in 2011 because they didn’t billion, easily eclipsing the record $33.1 Bankers are hopeful that this year will be like the valuations, but Wall Streeters say billion in 2000. But debt accounted for more stable, in which case investors might they are now running out of cash. As a 82% of the total dollars raised, compared be willing to support more deal flow dur- result, they will have to use the tricks at to only 19% in 2000 (see “Debt Domi- ing 1H12 (see “Fear Factor,” page 5). -

United States Court of Appeals for The
Case 14-4353, Document 100-1, 05/16/2016, 1772362, Page1 of 37 14‐4353‐cv Apotex Inc., et al., v. Acorda Therapeutics, Inc. UNITED STATES COURT OF APPEALS FOR THE SECOND CIRCUIT August Term, 2015 (Argued: November 12, 2015 Decided: May 16, 2016) Docket No. 14‐4353‐cv ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐x APOTEX INC., et al., Plaintiffs‐Appellants, ‐ v.‐ ACORDA THERAPEUTICS, INC., Defendant‐Appellee. ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐x Before: JACOBS, LIVINGSTON and DRONEY, Circuit Judges. This appeal concerns two distinct questions: the circumstances under which the filing of a citizen petition with the Food and Drug Administration provides grounds for an antitrust claim, and the scope of false advertising Case 14-4353, Document 100-1, 05/16/2016, 1772362, Page2 of 37 liability under the Lanham Act. Plaintiffs Apotex Incorporated and Apotex Corporation appeal from the judgment of the United States District Court for the Southern District of New York (Swain, J.) that granted defendant Acorda Therapeutics, Inc.’s motion to dismiss plaintiffs’ Sherman Act claim and that granted summary judgment in favor of defendant on the Lanham Act claims (Torres, J.). Because each of these conclusions was sound, we affirm. KEITH D. PARR (with Joseph N. Froehlich, Scott B. Feder, Hugh S. Balsam, James T. Peterka, and Andy J. Miller on the brief), Locke Lord LLP, Chicago, Illinois, for Appellants Apotex Incorporated & Apotex Corporation. JOHN W. NIELDS, JR. (with Jason C. Raofield and Colin P. Watson on the brief), Covington & Burling LLP, Washington, D.C. for Appellee Acorda Therapeutics, Inc. DENNIS JACOBS, Circuit Judge: The parties are rival manufacturers of tizanidine, a drug for treating spasticity. -

Q1 Pharma Sector Snapshot
SPECIALTY & GENERIC PHARMA Q1 2021 Report Market Commentary – Debt Capital Markets Debt Markets ▪ 2020 saw increased amounts of debt used in buyouts across the board, resulting in the highest debt / EBITDA Median US Buyout Multiples levels since 2014 − The increased use of debt was driven by 2H20 back- end loaded lending activity (primarily 4Q20) as 16.0x 12.7x 14.1x 12.2x 12.0x 11.6x 11.5x certainty around the U.S. election and vaccination 11.1x 10.0x 9.8x 12.0x 9.7x expectations increased 9.4x 8.6x 8.3x 8.2x 7.5x 7.8x 5.2x 6.7x 5.7x 5.6x ▪ 8.0x 5.9x As the effects of COVID now begin to diminish, debt 5.4x 4.4x 4.1x 3.7x 4.6x 4.3x 3.8x markets have seemingly recovered, signaling that 3.6x lenders have become increasingly comfortable with 4.0x 4.3x 6.9x 6.5x 6.3x 6.0x 5.9x 5.7x 5.7x 5.7x 5.7x 5.6x 5.3x 4.5x 4.4x macroeconomic and company-specific fundamentals 4.3x 0.0x 3.2x − With increased confidence, lenders are currently looking to provide strong leverage for high-quality assets, particularly ones that have proven their Debt/EBITDA Equity/EBITDA EV/EBITDA stability through the recent market downturn Source: PitchBook ▪ The spread on U.S. high-yield debt has returned to pre- Historical US High Yield Debt Effective Yield COVID levels − 4.22% current effective yield compared with a 12.0% 11.4% 11.38% effective yield on March 23, 2020 (peak of the pandemic) 9.0% ▪ We expect increased activity by lenders in 2021 due to: 6.0% 4.2% − Pent-up demand in M&A activity driven by the impact of COVID 3.0% − Limited Partner agreements and investor -

2020 Annual Report Products
Products 2020 Annual Report From the CEO Dear Fellow Shareholders, Perrigo’s transformation to a pure-play Consumer Self-Care Company has come a long way LQMXVWWZRVKRUW\HDUV:HKDYHUHVWRUHGVXVWDLQDEOHWRSOLQHJURZWKGHOLYHUHGRQRXU¿QDQFLDO SURPLVHVUHFRQ¿JXUHGRXUSRUWIROLRRIEXVLQHVVHVXSGDWHGWKH,7LQIUDVWUXFWXUHDQGSURFHVVHV of the Company, expanded capacity, upgraded leadership talent, installed business intelligence capabilities, built a new product pipeline of over $500 million and re-instilled a sense of pride and energy among our 11,000 team members. Making this even more remarkable, is that we kept the WUDQVIRUPDWLRQRQWUDFNLQWKHIDFHRIWKHJOREDO&29,'SDQGHPLF,KRSH\RXDUHDVSURXGRI3HUULJR¶VJOREDOWHDP DV,DPIRUKRZWKH\ZRUNHGWRNHHSHDFKRWKHUVDIHNHSWRXUHVVHQWLDOSURGXFWVÀRZLQJDQGNHSWRXUWUDQVIRUPDWLRQ to a consumer self-care company on track through all of the personal and professional uncertainty that came their way LQ7KH\DUHKHURHV As a result of their efforts, Perrigo delivered strong net sales growth for the second year in a row in 2020 and World-wide Consumer sales reached a new record high. Equally important, the team stabilized adjusted operating income after a few years of decline even as we invested over $50 million in our business and overcame $35 million RIXQSODQQHGKHDGZLQGVGXHSULPDULO\WR&29,'UHODWHGVDIHW\FRVWVDQGEXVLQHVVLPSDFWIURPWKHZHDNFROG FRXJKDQGÀXVHDVRQUHODWHGWR&29,'¶VLPSDFWRQSXEOLFOLIH$OOLQDOOZHKDGDYHU\VWURQJ\HDU Our transformation efforts reached an essential milestone after the year closed when we announced the sale of RXU3UHVFULSWLRQ3KDUPDFHXWLFDOVEXVLQHVVWR$OWDULV&DSLWDO3DUWQHUV//&7KHWUDQVDFWLRQUHLQIRUFHVRXUDELOLW\ -

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

DCAT MEMBER COMPANY MEETING LOCATOR V. 4
DCAT MEMBER COMPANY MEETING LOCATOR v. 4 THE BENJAMIN Sancilio Pharmaceuticals Company, Inc. Sri Krishna Pharmaceuticals Ltd. Amino Chemicals Ltd. Zydus Pharmaceuticals (USA) Inc. Apogee Pharma, Inc. C2 PHARMA INTERCONTINENTAL BARCLAY Calyx Chemicals & Pharmaceuticals Ltd. AbbVie* ChemCon GmbH ACIC Pharmaceuticals Inc. Concord Biotech Limited Advitech SA Dipharma Francis Srl Amneal Pharmaceuticals LLC DSM Sinochem Pharmaceuticals ALP Pharm F.I.S. - Fabbrica Italiana Sintetici S.p.A. AMRI Jost Chemical Co. Asymchem Inc. Jubilant Pharma Capsugel, Now a Lonza Company* PharmSource, A GlobalData Company CBC AMERICAS Corp. PolyPeptide Group CellMark USA, LLC ROHNER Inc. Charioteer Pharmaceutical Co., Ltd., Zhejiang FIFTY NYC, A AFFINA HOTEL Chemical and Pharmaceutical Solutions Chiral Quest Corp. Chartwell Pharmaceuticals, LLC Croda, Inc. HOTEL 48LEX DFE Pharma AB BioTechnologies, Inc. DPL-US AiPing Pharmaceutical, Inc. EQ Esteve Almac Evonik Corporation Aptuit LLC FAREVA SA AZAD Fine Chemicals Ltd. Flavine North America, Inc. Cambridge Isotope Laboratories, Inc. Formosa Laboratories, Inc. CMC Biologics Grifols International S.A. Groupe Parima Hainan Poly Pharm. Co., Ltd. Navin Fluorine International Limited Harris Pharmaceutical Qualicaps, Inc. Helm AG RC2 Pharma Connect LLC Hetero USA, Inc. Recipharm Hikal, Ltd. Recro Gainesville LLC Interchem Corporation Reed-Lane, Inc. Inventia Healthcare PVT LTD Please note: Some DCAT member companies have requested not to be listed in the locator. (*) indicates member companies with Business Meeting Spaces in more than one hotel. INTERCONTINENTAL BARCLAY CONT'D PiSA BioPharm, Inc. SPI Pharma Inc. Johnson Matthey Tapemark Kingchem Life Science LLC Unither Pharmaceutical Legacy Pharmaceutical Packaging Uquifa S.A. Lonza AG* Neuland Laboratories Ltd. LOTTE NY PALACE Orion Group AbbVie* Par Pharmaceutical, Inc. -

BTG INTERNATIONAL LIMITED V. AMNEAL PHARMACEUTICALS LLC
United States Court of Appeals for the Federal Circuit ______________________ BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMNEAL PHARMACEUTICALS LLC, AMNEAL PHARMACEUTICALS OF NEW YORK, LLC, DR. REDDY'S LABORATORIES, INC., DR. REDDY'S LABORATORIES, LTD., WOCKHARDT BIO AG, WOCKHARDT USA LLC, WOCKHARDT LTD., MYLAN PHARMACEUTICALS INC., MYLAN INC., WEST-WARD PHARMACEUTICALS CORP., NKA HIKMA PHARMACEUTICALS USA INC., HIKMA PHARMACEUTICALS LLC, TEVA PHARMACEUTICALS USA, INC., Defendants-Appellees PAR PHARMACEUTICAL, INC., PAR PHARMACEUTICAL COMPANIES, INC., RISING PHARMACEUTICALS, INC., Defendants ______________________ 2019-1147 ______________________ Appeal from the United States District Court for the District of New Jersey in Nos. 2:15-cv-05909-KM-JBC, 2:16-cv-02449-KM-JBC, 2:17-cv-06435-KM-JBC, Judge Kevin McNulty. 2 BTG INTERNATIONAL LIMITED v. AMNEAL PHARMACEUTICALS LLC - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - BTG INTERNATIONAL LIMITED, JANSSEN BIOTECH, INC., JANSSEN ONCOLOGY, INC., JANSSEN RESEARCH & DEVELOPMENT, LLC, Plaintiffs-Appellants v. AMERIGEN PHARMACEUTICALS, INC., AMERIGEN PHARMACEUTICALS LIMITED, Defendants-Appellees ______________________ 2019-1148 ______________________ Appeal from the United States District Court for the District of New Jersey in No. 2:16-cv-02449-KM-JBC, Judge Kevin McNulty. - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - JANSSEN -

COVID-19 Treatment and Vaccine Tracker This Document Contains an Aggregation of Publicly Available Information from Validated Sources
COVID-19 Treatment and Vaccine Tracker This document contains an aggregation of publicly available information from validated sources. It is not an endorsement of one approach or treatment over another but simply a list of all treatments and vaccines currently in development. TREATMENTS Current Type of FDA-Approved Clinical Trials for Funding Clinical Trials for Anticipated Next Number Developer/Researcher Stage of Published Results Sources Product - Treatment Indications Other Diseases Sources COVID-19 Steps Timing Development ANTIBODIES PhRMA Begin Phase 1 trials in late Polyclonal hyperimmune Alliance among Takeda, CSL Behring, Wall Street Journal spring. To patients between 1 globulin (H-IG), formerly N/A Biotest AG, Bio Products Laboratory, Pre-clinical Pink Sheet December 2020 and December known at TAK-888 LFB, and Octapharma Press release from the 2021 alliance Biomedical Stat News Advanced MarketWatch Antibodies from mice, Research and Reuters 2 REGN3048-3051, against the N/A Regeneron Pre-clinical Start Phase 1 June 2020 Development Bloomberg News spike protein Authority FierceBiotech (BARDA) FiercePharma Korea Herald Antibodies from recovered 3 N/A Celltrion Pre-clinical Start Phase 1 in July 2020 UPI COVID-19 patients Celltrion press release Super-antibody or antibody 4 cocktail to target potential N/A Celltrion Pre-clinical Celltrion press release mutations of SARS-CoV-2 Antibodies from recovered BioSpace 5 N/A Kamada Pre-clinical COVID-19 patients AbbVie Stat News Antibodies from recovered 6 N/A Vir Biotech/WuXi Biologics/Biogen Pre-clinical Start Phase 1 ~ July 2020 Vir Biotech COVID-19 patients Vir Biotech * Indicates updated or new field This document contains an aggregation of publicly available information from validated sources. -
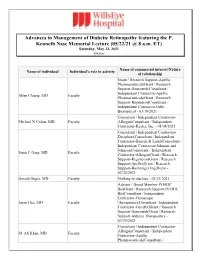
Advances in Management of Diabetic Retinopathy Featuring the P
Advances in Management of Diabetic Retinopathy featuring the P. Kenneth Nase Memorial Lecture (05/22/21 @ 8 a.m. ET) Saturday, May 22, 2021 Online Name of commercial interest/Nature Name of individual Individual's role in activity of relationship Grant / Research Support-Apellis Pharmaceuticals|Grant / Research Support-Genentech|Consultant / Independent Contractor-Apellis Allen Chiang, MD Faculty Pharmaceuticals|Grant / Research Support-Regeneron|Consultant / Independent Contractor-Orbit Biomedical - 03/19/2021 Consultant / Independent Contractor- Michael N Cohen, MD Faculty Allergan|Consultant / Independent Contractor-Keeler, Inc. - 05/04/2021 Consultant / Independent Contractor- Deciphera|Consultant / Independent Contractor-Bausch & Lomb|Consultant / Independent Contractor-Johnson and Johnson|Consultant / Independent Sunir J. Garg, MD Faculty Contractor-Allergan|Grant / Research Support-Regeneron|Grant / Research Support-Apellis|Grant / Research Support-Boehringer Ingelheim - 02/24/2021 Omesh Gupta, MD Faculty Nothing to disclose - 03/21/2021 Advisor / Board Member-IVERIC Bio|Grant / Research Support-IVERIC Bio|Consultant / Independent Contractor-Gyroscope Jason Hsu, MD Faculty Therapeutics|Consultant / Independent Contractor-OccuRx|Grant / Research Support-Genentech|Grant / Research Support-Aldeyra Therapeutics - 03/20/2021 Consultant / Independent Contractor- Allergan|Consultant / Independent M. Ali Khan, MD Faculty Contractor-Apellis Pharmaceuticals|Consultant / Independent Contractor- Genentech|Grant / Research Support- Regeneron