PDF Generation Date :- Mon, 09 Aug 2021 05:10:37
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
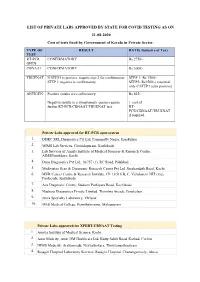
LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 21-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 21-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Private Labs approved for RT-PCR open system 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam 2. MIMS Lab Services, Govindapuram, Kozhikode 3. Lab Services of Amrita Institute of Medical Sciences & Research Centre, AIMSPonekkara, Kochi 4. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad 5. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Sreekandath Road, Kochi 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode 8. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam 9. Jeeva Specialty Laboratory, Thrissur 10. MES Medical College, Perinthalmanna, Malappuram Private Labs approved for XPERT/CBNAAT Testing 1. Amrita Institute of Medical Science, Kochi 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin 3. NIMS Medicity, Aralumoodu, Neyyattinkara, Thiruvananthapuram 4. Rajagiri Hospital Laboratory Services, Rajagiri Hospital, Chunangamvely, Aluva 5. Micro Health LAbs, MPS Tower, Kozhikode 6. Believers Church Medical College Laboratory, St Thomas Nagar, Kuttapuzha P.O., Thiruvalla 7. -
Annual Review Data for 2020.Xlsx
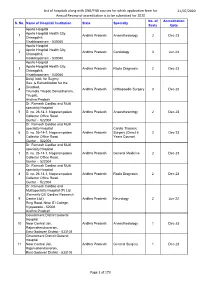
List of hospitals along with DNB/FNB courses for which application form for 21/07/2020 Annual Review of accreditation is to be submitted for 2020 No. of Accreditation S. No. Name of Hospital/ Institution State Specialty Seats Upto Apollo Hospital Apollo Hospital Health City, 1 Andhra Pradesh Anaesthesiology 2 Dec-23 Chinagdhili, Visakhapatnam - 530040 Apollo Hospital Apollo Hospital Health City, 2 Andhra Pradesh Cardiology 3 Jun-24 Chinagdhili, Visakhapatnam - 530040 Apollo Hospital Apollo Hospital Health City, 3 Andhra Pradesh Radio Diagnosis 2 Dec-23 Chinagdhili, Visakhapatnam - 530040 Balaji Instt. for Sugery Res. & Rehabilitation for the Disabled, 4 Andhra Pradesh Orthopaedic Surgery 3 Dec-23 Tirumala Tirupati Devasthanam, Tirupati, Andhra Pradesh Dr. Ramesh Cardiac and Multi speciality Hospital 5 D. no. 26-14-1, Nagarampalem Andhra Pradesh Anaesthesiology 2 Dec-23 Collector Office Road. Guntur - 522004 Dr. Ramesh Cardiac and Multi speciality Hospital Cardio Thoracic 6 D. no. 26-14-1, Nagarampalem Andhra Pradesh Surgery (Direct 6 2 Dec-23 Collector Office Road. Years Course) Guntur - 522004 Dr. Ramesh Cardiac and Multi speciality Hospital 7 D. no. 26-14-1, Nagarampalem Andhra Pradesh General Medicine 2 Dec-23 Collector Office Road. Guntur - 522004 Dr. Ramesh Cardiac and Multi speciality Hospital 8 D. no. 26-14-1, Nagarampalem Andhra Pradesh Radio Diagnosis 2 Dec-23 Collector Office Road. Guntur - 522004 Dr. Ramesh Cardiac and Multispeciality Hospital (P) Ltd. (Formerly Citi Cardiac Research 9 Centre Ltd.) Andhra Pradesh Neurology 2 Jun-22 Ring Road, Near ITI College, Vijayawada - 52008 Andhra Pradesh Government District General Hospital 10 Near Central Jail, Andhra Pradesh Anaesthesiology 1 Dec-23 Rajamahendravaram, East Godavari District - 533105 Government District General Hospital 11 Near Central Jail, Andhra Pradesh General Surgery 1 Dec-23 Rajamahendravaram, East Godavari District - 533105 Page 1 of 173 List of hospitals along with DNB/FNB courses for which application form for 21/07/2020 Annual Review of accreditation is to be submitted for 2020 No. -

S.No. Name of Ethics Committee RC No
S.No. Name of Ethics Committee RC No. Address State All India Institute of Medical Sciences, Room No.102, 1st floor, OT Block, 1 Ethics Committee ECR/538/Inst/DL/2014/RR-17 Delhi Ansari Nagar, New Delhi-110029 Aster Aadhar Hospital, R.S. NO. 628, 'B' Ward, Near Shastri Nagar, KMT 2 Aster Aadhar Ethics Committee ECR/470/Inst/MH/2013/RR-16 Maharashtra Workshop, Kohlapur-416012 3 Visakha Institutional Review Board ECR/4/Indt/AP/2013/RR-16 A-11, Prince Villae Royal Appartment, Siripuram, Visakhapatnam- 530003 Andhra Pradesh SAUMYAA, C-321, Behind Ganesh Temple N-1, CIDCO, Aurangabad- 4 Aurangabad Ethics Committee ECR/122/Indt/MH/2013/RR-16 Maharashtra 431003 Ethics Committee of Diabetes Thyroid BCM Health Island, PU4, Scheme 54, Behind Prestige Management 5 ECR/409/Inst/MP/2013/RR-16 Madhya Pradesh Hormone Research Institute Institute, Near Bombay Hospital, Indore- 452010 Jehangir Clinical Development Centre Pvt. Ltd., Jehangir Hospital 6 Ethics Committee ECR/352/Inst/MH/2013/RR-19 Maharashtra Premises, 32 Sassoon Road, Pune- 411001 89, 3rd Cross, S V K Layout, Basaveshwar Nagar, Banagalore, Urban- 7 Lifeline Ethics Committee ECR/76/Indt/KA/2013/RR-16 Karnataka 560079 Instutional Ethics Committee-Clinical Indraprastha Apollo Hospitals, Sarita Vihar, Delhi- Mathura road, New 8 ECR/5/Inst/DL/2013/RR-16 Delhi Studies Delhi- 110076 H.No.- D/129, St. No.-13 Opp-Durga Mandir, Ashok Nagar, Shahdara- 9 Good Society for Ethical Research ECR/69/Indt/DL/2013/RR-19 Delhi 110093 Ethics Committee, S.P. Medical College & S.P Medical College & A.G Hospitals, HRMC Cardiovascular Sciences & 10 ECR/27/SP/Inst/RJ/2013/RR-16 Rajasthan A.G. -

Page 1 of 54 List of NABL Accredited Laboratories for RT PCR RNA
List of NABL Accredited Laboratories for RT PCR RNA (updated as on 29-07-2021) Total 1490 Labs (Private + Government + International) PRIVATE LABORATORIES (1472 accredited for RT PCR RNA;1421 Labs listed by ICMR) Sl. State City Sl. Name of the laboratory Certificate. Valid Upto ICMR listed No No. No. 1421 (as on 28-07-2021) 1. Andhra Pradesh Anantapur 1. Care & Cure Molecular Biology Laboratory MC-3760 02-10-2022 ICMR listed (52) Amalapuram 2. Konaseema Institute of Medical Sciences and Research MC-3925 22-11-2022 ICMR listed Foundation (A Unit of Mother Theressa Educational Society) Eluru 3. ASRAM Central Laboratory, Alluri Sitarama Raju Academy of MC-2501 17-12-2021 ICMR listed Medical Sciences (ASRAM) Chittoor 4. Department of Microbiology- Apollo Institute of Medical MC-3776 06-10-2022 ICMR listed Sciences and Research (Apollo Medical Centre) A Unit of Apollo Hospitals Educational and Research Foundation Cuddapah 5. Fathima Institute of Medical Sciences, Molecular Laboratory, MC-3881 03-11-2022 ICMR listed A Unit of Mohammadiya Educational Society Cuddapah 6. SRS Diagnostics MC-4165 18-02-2023 ICMR listed Guntur 7. Department of Laboratory Medicine, Manipal Hospital, A Unit MC-2005 26-02-2022 ICMR listed of Manipal Hospitals (Jaipur) Private Limited Guntur 8. Yontus Life Sciences Pvt. Ltd. MC-3654 09-09-2022 ICMR listed Guntur 9. Milestone Labs MC-3671 12-09-2022 ICMR listed Guntur 10. Lab Services, NRI General Hospital MC-3045 11-10-2022 ICMR listed Guntur 11. Konacc Diagnostic Centre (A Unit of Konacc Diagnostics MC-4012 13-12-2022 ICMR listed Private Limited) Guntur 12. -
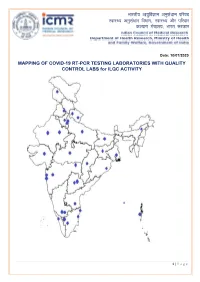
MAPPING of COVID-19 RT-PCR TESTING LABORATORIES with QUALITY CONTROL LABS for ILQC ACTIVITY
भारतीय आयुर्विज्ञान अनुसंधान पर्रषद वा्य अनुसंधान र्वभाग, वा्य और पर्रवार क쥍याण मंत्रालय, भारत सरकार Date: 10/07/2020 MAPPING OF COVID-19 RT-PCR TESTING LABORATORIES WITH QUALITY CONTROL LABS for ILQC ACTIVITY 1 | P a g e QC labs across India State Name of QC Lab Telangana Gandhi Medical College, Secunderabad Osmania Medical College, Hyderabad Andhra Pradesh Sri Venkateshwara Institute of Medical Sciences, Tirupati Uttar Pradesh King George Medical University, Lucknow Uttarakhand All India Institute of Medical Sciences, Rishikesh Govt. Medical College, Haldwani West Bengal National Institute of Cholera and Enteric Diseases, Kolkata Assam, Arunachal Pradesh, Manipur, Regional Medical Research Centre, Dibrugarh Meghalaya, Mizoram, Nagaland, Tripura Bihar ICMR-Rajendra Memorial Research Institute of Medical Sciences, Patna Chandigarh, Himachal Pradesh and Post Graduate Institute of Medical Education & Research, Punjab Chandigarh Chhattisgarh All India Institute of Medical Sciences, Raipur Delhi All India Institute Medical Sciences, Delhi Maulana Azad Medical College, Delhi Gujarat BJ Medical College, Ahmedabad Maharashtra ICMR-National Institute of Virology, Pune ICMR-National Institute of Virology Field Unit, Mumbai Kasturba Hospital for Infectious Diseases, Mumbai All India Institute of Medical Sciences, Nagpur Haryana BPS Govt. Medical College, Sonipat Ladakh Sher-i-Kashmir Institute of Medical Sciences, Srinagar Jharkhand Rajendra Institute of Medical Sciences, Ranchi Karnataka Bangalore Medical College & Research Institute, Bengaluru ICMR-National Institute of Virology, Bangalore Field Unit, Bengaluru Kerala ICMR-National Institute of Virology, Field Unit, Allapuzzha Madhya Pradesh All India Institute of Medical Sciences, Bhopal Odisha ICMR-Regional Medical Research Centre, Bhubaneshwar Puducherry Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry Rajasthan Sawai Man Singh Medical College, Jaipur All India Institute of Medical Sciences, Jodhpur Tamil Nadu King Institute of Preventive Medicine & Research, Chennai Govt. -

SR No HOSPITAL NAME
HOSPITAL NAME (Hospitals marked with** S R are Preffered Provider STD Telephon Address City Pin Rohini Code State Zone No Network, with whom ITGI code e has Negotiated package rates) 1 Aasha Hospital** 7-201, Court Road Anantapur 515001 08554 245755 8900080169586 Andhra Pradesh South Zone 2 Aayushman The Family Hospital**45/142A1, V.R. ColonyKurnool 518003 08518 254004 8900080172005 Andhra Pradesh South Zone 3 Akira Eye Hospital** Aryapuram Rajahmundry 533104 0883 2471147 8900080180079 Andhra Pradesh South Zone 4 Andhra Hospitals** C.V.R. Complex, PrakasamVijayawada Road 520002 0866 2574757 8900080172531 Andhra Pradesh South Zone 5 Apex Hospital # 75-6-23, PrakashnagarRajahmundry Rajahmundry 533103 0883 2439191 8900080334724 Andhra Pradesh South Zone 6 Apollo Bgs Hospitals** Adichunchanagari RoadMysore Kuvempunagar 570023 0821 2566666 8900080330627 Karnataka South Zone 7 Apollo Hospital 13-1-3, Suryaraopeta, MainKakinada Road 533001 0884 2379141 8900080341647 Andhra Pradesh South Zone 8 Apollo Hospitals,Vizag Waltair, Main Road Visakhapatnam 530002 0891 2727272 8900080177710 Andhra Pradesh South Zone 9 Apoorva Hospital 50-17-62 Rajendranagar,Visakhapatnam Near Seethammapeta530016 Jn. 0891 2701258 8900080178007 Andhra Pradesh South Zone 10 Aravindam Orthopaedic Physiotherapy6-18-3, KokkondavariCentre Street,Rajahmundry T. Nagar, Rajahmundry533101 East0883 Godavari2425646 8900080179547 Andhra Pradesh South Zone 11 Asram Hospital (Alluri Sitarama N.H.-5,Raju Academy Malaka OfPuram MedicalEluru Sciences)** 534005 08812 249361-62 8900080180895 -

Noorul Islam Centre for Higher Education-NAAC
Noorul Islam Centre for Higher Education-NAAC SSR Noorul Islam Centre for Higher Education (Deemed to be University u/s 3 of the UGC Act 1956) Kumaracoil-629180, Thuckalay, Kanyakumari District., Tamilnadu Ph: 04651-250467, Mobile: 9444022063 E-Mail: [email protected] website: www.niceindia.com Prof. Dr.R.Perumalsamy 17.09.2015 Vice Chancellor The Director National Assessment and Accreditation Council Bangalore – 560 072 Sir, Sub: NAAC Accreditation Submission of SSR – Reg. Ref: Your e-mail dated 02.07.2015 ------ Greetings from Noorul Isalm Centre for Higher Education. With reference to your e-mail dated 2nd July 2015, Noorul Islam Centre for Higher Education is sending herewith EIGHT COPIES of “SELF STUDY REPORT” by Courier /Speed Post. Self Study Report has also been duly uploaded in the University website. Evidence for having uploaded data in the MHRD website (http://aishe.gov.in) is also annexed to the SSR. A Syndicate Bank Draft No.386956 dated 17.09.2015 for Rs.6,84,000/- (Rupees Six lakhs eighty four thousand only) drawn in favour of the Director, NAAC, payable at Bangalore has been enclosed towards Accreditation Fee. Thanking you, Yours faithfully, (R.PERUMALSAMY) Encl: As above. Covering Letter Noorul Islam Centre for Higher Education-NAAC SSR Contents Sr. No. PARTICULARS Page No. A Covering Letter B Executive Summary E1-E10 SWOC & Future Plan S1-S3 C Profile of the University P1-P11 D Criteria-wise Inputs Criterion I: Curricular Aspects C1-C19 Criterion II: Teaching-Learning and Evaluation C20-C66 Criterion III: Research, Consultancy and C67-C115 Extension Criterion IV: Infrastructure and Learning C116-C134 Resources Criterion V: Student Support and Progression C135-C159 Criterion VI: Governance, Leadership And C160-C175 Management Criterion VII: Innovations and Best Practices C176-C183 E Evaluative Report of the department 1. -

LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 13-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 13-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Private Labs approved for RT-PCR open system 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam 2. MIMS Lab Services, Govindapuram, Kozhikode 3. Lab Services of Amrita Institute of Medical Sciences & Research Centre, AIMSPonekkara, Kochi 4. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad 5. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Sreekandath Road, Kochi 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode 8. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam 9. Jeeva Specialty Laboratory, Thrissur Private Labs approved for XPERT/CBNAAT Testing 1. AIMS, Kochi 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin 3. NIMS Medicity, Aralumoodu, Neyyattinkara, Thiruvananthapuram 4. Rajagiri Hospital Laboratory Services, Rajagiri Hospital, Chunangamvely, Aluva 5. Micro Health LAbs, MPS Tower, Kozhikode 6. Believers Church Medical College Laboratory, St Thomas Nagar, Kuttapuzha P.O., Thiruvalla 7. Al Salama Diagnostic Centre, Thalakkadathur, Tirur (P) 161. -

Total No. of Labs : 2552
भारतीय आयु셍वज्ञि ान अनुसंधान पररषद वा्य अनुसंधान 셍वभाग, वा्य और पररवार क쥍याण मंत्रालय, भारत सरकार Indian Council of Medical Research Department of Health Research, Ministry of Health and Family Welfare, Government of India Date: 18/05/2021 Total Operational (initiated independent testing) Laboratories reporting to ICMR: Government laboratories : 1256 Private laboratories : 1296 - Real-Time RT PCR for COVID-19 : 1497 (Govt: 582 + Private: 915) - TrueNat Test for COVID-19 : 916 (Govt: 629 + Private: 287) - CBNAAT Test for COVID-19 : 132 (Govt: 43 + Private: 89) - Other Molecular-Nucleic Acid (M-NA) Testing Platforms for COVID-19 : 07 (Govt: 02 + Private: 05) Note: Other Molecular-Nucleic Acid includes Abbott ID NOW, RT-LAMP and CRISPR-Cas9 Total No. of Labs : 2552 *CSIR/DBT/DST/DAE/ICAR/DRDO/MHRD/ISRO Laboratories. #Laboratories approved for both Real-Time RT-PCR and TrueNat/CBNAAT $Laboratories approved for both TrueNAT and CBNAAT ¥ Laboratories approved for Abbott ID NOW alone or in combination with any other testing platforms @Laboratories approved for RT-LAMP alone or in combination with any other testing platforms € Laboratories approved for CRISPR-Cas9 alone or in combination with any other testing platforms P: Provisional Δ Pvt. Laboratories acquired by Govt. 1 | P a g e S. Test Names of States Names of Government Institutes Names of Private Institutes No. Category 1. Andhra Pradesh RT-PCR 1. Sri Venkateswara Institute of Medical 1. Manipal Hospital, Tadepalli, Guntur (126) Sciences, Tirupati 2. PathGene Health Care Pvt Ltd#2nd Floor, 2. Sri Venkateswara Medical College, Srinivasapuram, Tiruchanoor Road, Opp LV Govt: 78 Tirupati kayanamandapam, Tirupathi Private: 48 3. -
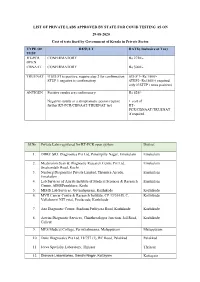
LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 29-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 29-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Sl.No Private Labs registered for RT-PCR open system District 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam Ernakulam 2. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Ernakulam Sreekandath Road, Kochi 3. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam Ernakulam 4. Lab Services of Amrita Institute of Medical Sciences & Research Ernakulam Centre, AIMSPonekkara, Kochi 5. MIMS Lab Services, Govindapuram, Kozhikode Kozhikode 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Kozhikode Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode Kozhikode 8. Aswini Diagnostic Services, Chinthavalappu Junction, Jail Road, Kozhikode Calicut 9. MES Medical College, Perinthalmanna, Malappuram Malappuram 10. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad Palakkad 11. Jeeva Specialty Laboratory, Thrissur Thrissur 12. Dianova Laboratories, Gandhi Nagar, Kottayam Kottayam Sl.No Private Labs registered for XPERT Testing District 1. Amrita Institute of Medical Science, Kochi Ernakulam 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin Ernakulam 3. -
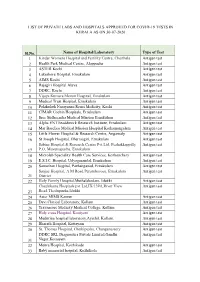
List of Private Labs and Hospitals Approved for Covid-19 Tests in Kerala As on 30-07-2020
LIST OF PRIVATE LABS AND HOSPITALS APPROVED FOR COVID-19 TESTS IN KERALA AS ON 30-07-2020 Sl.No. Name of Hospital/Laboratory Type of Test 1 Kinder Womens Hospital and Fertility Centre, Cherthala Antigen test 2 Health Park Medical Centre, Alappuzha Antigen test 3 ASTER Kochi Antigen test 4 Lakeshore Hospital, Ernakulam Antigen test 5 AIMS Kochi Antigen test 6 Rajagiri Hospital Aluva Antigen test 7 DDRC, Kochi Antigen test 8 Vijaya Kumara Menon Hospital, Ernakulam Antigen test 9 Medical Trust Hospital, Ernakulam Antigen test 10 Polakulath Narayanan Renai Medicity, Kochi Antigen test 11 CIMAR Cochin Hospitals, Ernakulam Antigen test 12 Sree Sudheendra Medical Mission Ernakulam Antigen test 13 Alpha ENT head&neck Research Institute, Ernakulam Antigen test 14 Mar Baselios Medical Mission Hospital Kothamangalam Antigen test 15 Little Flower Hospital & Research Centre, Angamaly Antigen test 16 St Joseph Hospital, Dharmagiri, Ernakulam Antigen test Sabine Hospital & Research Centre Pvt Ltd, Pezhakkappilly Antigen test 17 P.O, Muvattupuzha, Ernakulam 18 Microlab Speciality Health Care Services, Kozhenchery Antigen test 19 E.S.I.C. Hospital, Udyogmandal, Ernakulam Antigen test 20 Samaritan Hospital, Pazhanganad, Ernakulam Antigen test Sanjoe Hospital, A M Road Perumbavoor, Ernakulam Antigen test 21 District 22 Holy Family Hospital,Muthalakodam, Idukki Antigen test Chazhikattu Hospitals pvt Ltd,IX/139A,River View Antigen test 23 Road,Thodupuzha,Idukki 24 Aster MIMS Kannur Antigen test 25 Devi Clinical Laboratory, Kollam Antigen test 26 Travancore Medicity Medical College, Kollam Antigen test 27 Holy cross Hospital, Kottiyam Antigen test 28 Meditrina hospital laboratory,Ayathil, Kollam, Antigen test 29 Bharath Hospital, Kottayam Antigen test 30 St. -
BDS University 2Nd and 3Rd Ranks in 2015
NOORUL ISLAM COLLEGE OF DENTAL SCIENCE NIMS MEDICITY ARALUMMOODU, NEYYATTINKARA -695 123 THIRUVANANTHAPURAM DISTRICT, KERALA STATE APPROVED BY DENTAL COUNCIL OF INDIA AFFILIATED TO KERALA UNIVERSITY OF HEALTH SCIENCE, TRISSUR PHONE: 0471- 2226513, 2221546, FAX: 0471 -2225154 E-mail : [email protected] website: www.nicollegeofdentalscience.com PROSPECTUS 2016-2017 3 Preface Noorul Islam College of Dental Science was established under the aegis of Noorul Islam Educational Trust, founded by Dr.A.P Majeed Khan, a man renowned for his farsighted vision in the field of education. This institute is well equipped to provide graduate and Post Graduate courses in Dentistry and is a breakthrough in dental education and health care facilities in Kerala. Through a short spam of 10 years Noorul Islam College of Dental Science has attained excellence in dental science by bagging 28 ranks in the university, which includes final BDS university 2nd and 3rd ranks in 2015. The beautifully landscaped campus is located in close proximity to Trivandrum and Kanya Kumari. Housed in this sprawling campus are the most modern clinical and lecture hall facilities, state‐of‐the‐art equipments, a well‐stocked library and separate hostels for boys and girls. Noorul Islam College of Dental Science is affiliated to the Kerala University of Health Sciences and recognized by the Dental Council of India. Noorul Islam college of Dental Science, NIMS Medicity, part of the 60 year old Noorul Islam Educational Trust, has a 350 bedded Super specialty Hospital, at Neyyattinkara, Trivandrum. It is one of the largest health care centers in South Kerala and South Tamilnadu.