XERODERMA PIGMENTOSUM: a MULTIDISCIPLINARY APPROACH Mieran Sethi,1 Alan R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Risk of Cancer in Patients with Psoriasis a Population-Based Cohort Study in the Health Improvement Network
Research Original Investigation The Risk of Cancer in Patients With Psoriasis A Population-Based Cohort Study in the Health Improvement Network Zelma C. Chiesa Fuxench, MD; Daniel B. Shin, MS; Alexis Ogdie Beatty, MD, MSCE; Joel M. Gelfand, MD, MSCE IMPORTANCE The risk of cancer in patients with psoriasis remains a cause of special concern due to the chronic inflammatory nature of the disease, the use of immune-suppressive treatments and UV therapies, and the increased prevalence of comorbid, well-established risk factors for cancer, such as smoking and obesity, all of which may increase the risk of carcinogenesis. OBJECTIVE To compare the overall risk of cancer, and specific cancers of interest, in patients with psoriasis compared with patients without psoriasis. DESIGN, SETTING, AND PARTICIPANTS Population-based cohort study of patients ages 18 to 89 years with no medical history of human immunodeficiency virus, cancer, organ transplants, or hereditary disease (albinism and xeroderma pigmentosum), prior to the start date, conducted using The Health Improvement Network, a primary care medical records database in the United Kingdom. The data analyzed had been collected prospectively from 2002 through January 2014. The analysis was completed in August 2015. EXPOSURES OF INTEREST Patients with at least 1 diagnostic code for psoriasis were classified as having moderate-to-severe disease if they had been prescribed psoralen, methotrexate, cyclosporine, acitretin, adalimumab, etanercept, infliximab, or ustekinumab or phototherapy for psoriasis. Patients were classified as having mild disease if they never received treatment with any of these agents. MAIN OUTCOMES AND MEASURES Incident cancer diagnosis. RESULTS A total of 937 716 control group patients without psoriasis, matched on date and practice visit, and 198 366 patients with psoriasis (186 076 with mild psoriasis and 12 290 with moderate-to-severe disease) were included in the analysis. -

Jebmh.Com Original Research Article
Jebmh.com Original Research Article OCULAR INVOLVEMENT IN MUCOCUTANEOUS DISORDERS- A STUDY IN TERTIARY HOSPITAL IN SOUTH ORISSA Sarita Panda1, B. N. R. Subudhi2, Prangya Panda3, Santosh Kumar Padhi4 1Assistant Professor, Department of Ophthalmology, M.K.C.G. Medical College, Berhampur, Orissa. 2Professor and HOD, Department of Ophthalmology, M.K.C.G. Medical College, Berhampur, Orissa. 3Associate Professor, Department of Ophthalmology, M.K.C.G. Medical College, Berhampur, Orissa. 4Senior Medical Officer, Government City Hospital, Berhampur, Orissa. ABSTRACT BACKGROUND Diseases of skin, mucous membrane and mucocutaneous junctions may also affect the eyes. Physical findings of dermatological disorders and eyes overlap due to three factors- (i) Genodermatoses often affects both skin and eyes because of origin from embryonic ectodermal layers, (ii) Acquired dermatological disorders may affect the mucocutaneous tissue of periorbital regions, (iii) Systemic diseases can manifest as diseases of skin and periocular mucocutaneous tissue because of their superficial anatomical locations. The aim of the present study was observation and interpretation of changes in the eye in different mucocutaneous disorders and correlation of the eye changes with severity of the diseases. MATERIALS AND METHODS A prospective study was undertaken in the Department of Ophthalmology, M.K.C.G. Medical College and Hospital, Berhampur, South Orissa, during the period of 2014 to 2016 including the referred patients after being diagnosed with mucocutaneous disease from Department of Dermatology, Paediatric and Medicine from the same hospital. A case study of 204 patients (M- 164, F-40) was done. All patients underwent detailed ophthalmic examination inclusive of ocular movements, VA, IOP, S/L exam, blood and urine investigation and fundus examination. -

Table S1. Checklist for Documentation of Google Trends Research
Table S1. Checklist for Documentation of Google Trends research. Modified from Nuti et al. Section/Topic Checklist item Search Variables Access Date 11 February 2021 Time Period From January 2004 to 31 December 2019. Query Category All query categories were used Region Worldwide Countries with Low Search Excluded Volume Search Input Non-adjusted „Abrasion”, „Blister”, „Cafe au lait spots”, „Cellulite”, „Comedo”, „Dandruff”, „Eczema”, „Erythema”, „Eschar”, „Freckle”, „Hair loss”, „Hair loss pattern”, „Hiperpigmentation”, „Hives”, „Itch”, „Liver spots”, „Melanocytic nevus”, „Melasma”, „Nevus”, „Nodule”, „Papilloma”, „Papule”, „Perspiration”, „Petechia”, „Pustule”, „Scar”, „Skin fissure”, „Skin rash”, „Skin tag”, „Skin ulcer”, „Stretch marks”, „Telangiectasia”, „Vesicle”, „Wart”, „Xeroderma” Adjusted Topics: "Scar" + „Abrasion” / „Blister” / „Cafe au lait spots” / „Cellulite” / „Comedo” / „Dandruff” / „Eczema” / „Erythema” / „Eschar” / „Freckle” / „Hair loss” / „Hair loss pattern” / „Hiperpigmentation” / „Hives” / „Itch” / „Liver spots” / „Melanocytic nevus” / „Melasma” / „Nevus” / „Nodule” / „Papilloma” / „Papule” / „Perspiration” / „Petechia” / „Pustule” / „Skin fissure” / „Skin rash” / „Skin tag” / „Skin ulcer” / „Stretch marks” / „Telangiectasia” / „Vesicle” / „Wart” / „Xeroderma” Rationale for Search Strategy For Search Input The searched topics are related to dermatologic complaints. Because Google Trends enables to compare only five inputs at once we compared relative search volume of all topics with topic „Scar” (adjusted data). Therefore, -

Diagnosis and Management of Cutaneous Psoriasis: a Review
FEBRUARY 2019 CLINICAL MANAGEMENT extra Diagnosis and Management of Cutaneous Psoriasis: A Review CME 1 AMA PRA ANCC Category 1 CreditTM 1.5 Contact Hours 1.5 Contact Hours Alisa Brandon, MSc & Medical Student & University of Toronto & Toronto, Ontario, Canada Asfandyar Mufti, MD & Dermatology Resident & University of Toronto & Toronto, Ontario, Canada R. Gary Sibbald, DSc (Hons), MD, MEd, BSc, FRCPC (Med Derm), ABIM, FAAD, MAPWCA & Professor & Medicine and Public Health & University of Toronto & Toronto, Ontario, Canada & Director & International Interprofessional Wound Care Course and Masters of Science in Community Health (Prevention and Wound Care) & Dalla Lana Faculty of Public Health & University of Toronto & Past President & World Union of Wound Healing Societies & Editor-in-Chief & Advances in Skin and Wound Care & Philadelphia, Pennsylvania The author, faculty, staff, and planners, including spouses/partners (if any), in any position to control the content of this CME activity have disclosed that they have no financial relationships with, or financial interests in, any commercial companies pertaining to this educational activity. To earn CME credit, you must read the CME article and complete the quiz online, answering at least 13 of the 18 questions correctly. This continuing educational activity will expire for physicians on January 31, 2021, and for nurses on December 4, 2020. All tests are now online only; take the test at http://cme.lww.com for physicians and www.nursingcenter.com for nurses. Complete CE/CME information is on the last page of this article. GENERAL PURPOSE: To provide information about the diagnosis and management of cutaneous psoriasis. TARGET AUDIENCE: This continuing education activity is intended for physicians, physician assistants, nurse practitioners, and nurses with an interest in skin and wound care. -

Skin Signs of Rheumatic Disease Gideon P
Skin Signs of Rheumatic Disease Gideon P. Smith MD PhD MPH Vice Chair for Clinical Affairs Director of Rheumatology-Dermatology Program Director of Connective Tissue Diseases Fellowship Associate Director of Clinical Trials Department of Dermatology Massachusetts General Hospital Harvard University www.mghcme.org Disclosures “Neither I nor my spouse/partner has a relevant financial relationship with a commercial interest to disclose.” www.mghcme.org CONNECTIVE TISSUE DISEASES CLINIC •Schnitzlers •Interstitial •Chondrosarcoma •Eosinophilic Fasciitis Granulomatous induced •Silicone granulomas Dermatitis with Dermatomyositis Arthritis •AML arthritis with •Scleroderma granulomatous papules •Cutaneous Crohn’s •Lyme arthritis with with arthritis •Follicular mucinosis in papular mucinosis JRA post-infliximab •Acral Anetoderma •Celiac Lupus •Calcinosis, small and •Granulomatous exophytic •TNF-alpha induced Mastitis sarcoid •NSF, Morphea •IgG4 Disease •Multicentric Reticul •EED, PAN, DLE ohistiocytosis www.mghcme.org • Primary skin disease recalcitrant to therapy Common consults • Hair loss • Nail dystrophy • Photosensitivity • Cosmetic concerns – post- inflammatory pigmentation, scarring, volume loss, premature photo-aging • Erythromelalgia • Dry Eyes • Dry Mouth • Oral Ulcerations • Burning Mouth Syndrome • Urticaria • Itch • Raynaud’s • Digital Ulceration • Calcinosis cutis www.mghcme.org Todays Agenda Clinical Presentations Rashes (Cutaneous Lupus vs Dermatomyositis vs ?) Hard Skin (Scleroderma vs Other sclerosing disorders) www.mghcme.org -

A Comprehensive Review of Acne Vulgaris AK Mohiuddin1* 1Department of Pharmacy, World University of Bangladesh
Symbiosis ISSN Online: 2378-1726 www.symbiosisonlinepublishing.com Review Article Clinical Research in Dermatology: Open Access Open Access A Comprehensive Review of Acne Vulgaris AK Mohiuddin1* 1Department of Pharmacy, World University of Bangladesh Received: May 25, 2019; Accepted: June 6, 2019; Published: June 17, 2019 *Corresponding author: AK Mohiuddin, Assistant Professor, Department of Pharmacy, World University of Bangladesh, 151/8, Green Road, Dhanmondi, Dhaka – 1205, Bangladesh. E-mail: [email protected]; Orcid Id: https://orcid.org/0000-0003-1596-9757. Abstract Acne, also known as acne vulgaris (AV), is a long-term skin disease that occurs when hair follicles are clogged with dead skin cells and oil from the skin. It is characterized by blackheads or whiteheads, pimples, oily skin, and possible scarring. An intact stratum corneum and barrier, normal natural moisturizing factor and hyaluronic acid levels, normal Aquaporin-3 (AQP3) expression (localized at the basal lateral membranes of collecting duct cells in the kidney), and balanced sebum secretion are qualities of the skin that fall in the middle of the oily–dry spectrum. Patients rarely, if ever, complain about reduced sebum production, but elevated sebum production, yielding oily skin that can be a precursor to acne, is a common Propionibacterium acnes in adolescence, under the complaint. Several factors are known to influence sebum production. AV is mostly triggered by impact,influence as of sebum normal levels circulating are usually dehydroepiandrosterone low in childhood, rise (DHEA). in the middle-to-late It is a very common teen years, skin disorder and remain which stable can presentinto the withseventh inflammatory and eighth and decades non- untilinflammatory endogenous lesions androgen chiefly synthesis on the face dwindles. -

Review Article Stevens-Johnson Syndrome Basak AK1, Debnath J2
KYAMC Journal Vol. 8, No.-2, January 2018 Review Article Stevens-Johnson Syndrome Basak AK1, Debnath J2 Background Stevens-Johnson syndrome (SJS) is an immune complex mediated hypersensitivity complex that typically involves the skin and mucous membranes. While minor presentations may occur, significant involvement of oral, nasal, eye, vaginal, urethral, gastrointestinal, and lower respiratory mucous membranes may develop in the course of the illness. GI and respiratory involvement may progress to necrosis. Stevens-Johnson syndrome is a serious systemic disorder with the potential for severe morbidity and even death. The syndrome was first described in 1922, when the American pediatricians Albert Mason Stevens and Frank Chambliss Johnson reported the cases of 2 boys aged 7 and 8 years with "an extraordinary, generalized eruption with continued fever, inflamed buccal mucosa, and severe purulent conjunctivitis". Both cases had been misdiagnosed by primary care physicians as hemorrhagic measles. Erythema multiforme (EM), originally described by Von Hebra in 1866, was part of the differential diagnosis in both cases but was excluded because of the 'character of skin lesions, the lack of subjective symptoms, the prolonged high fever, and the terminal heavy crusting". Despite the presence of leucopenia in both cases, Stevens and Johnson in their initial report suspected an infectious disease of unknown etiology as the cause. In 1950, Thomas divided EM into 2 catagories: erythema multiforme minor (Von Hebra) and Erythema multiforme major (EMM). Since 1983, erythema multiforme major and Stevens-Johnson syndrome had been considered synonymous. In the 1990s, however, Bastuji and Roujeau each proposed that Erythema multiforme major and Stevens-Johnson syndrome are 2 distinct disorders. -

Skin Disorders in Diabetes Mellitus: an Epidemiology and Physiopathology Review Geisa Maria Campos De Macedo1*, Samanta Nunes2 and Tania Barreto3
de Macedo et al. Diabetol Metab Syndr (2016) 8:63 DOI 10.1186/s13098-016-0176-y Diabetology & Metabolic Syndrome REVIEW Open Access Skin disorders in diabetes mellitus: an epidemiology and physiopathology review Geisa Maria Campos de Macedo1*, Samanta Nunes2 and Tania Barreto3 Abstract Skin disorders, usually neglected and frequently underdiagnosed among diabetic patients, are common complica- tions and encounter a broad spectrum of disorders in both type 1 and type 2 diabetes mellitus (DM)—e.g. cutaneous infection, dry skin, pruritus. Skin disorders are highly associated with increased risk of important outcomes, such as skin lesions, ulcerations and diabetic foot, which can lead to major complications and revolve around multifactorial factors besides hyperglycemia and advanced glycation end products. Although diabetic’s skin disorders are consistent in the literature, there is limited data regarding early-stage skin disorders in DM patients. Disease control, early-stage treatment (e.g. skin hydration, orthotic devices) and awareness can reduce morbidity of DM patients. Thus, better understanding of the burden of skin disorders in DM patients may raise awareness on prevention and management. Therefore, the aim of this study is to perform a literature review to evaluate the main clinical characteristics and com- plications of skin disorders in diabetic’s patients. Additionally, physiopathology early-stage skin disorders and dermo- cosmetic management were also reviewed. Keywords: Diabetes mellitus, Skin disorders, Non-pharmacological treatment Background Skin disorders in diabetes and epidemiology Skin disorders, usually neglected and frequently under- Diabetes mellitus (DM) represents a high prevalent dis- diagnosed among diabetic patients, are common com- ease with high morbidity and mortality. -

Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group
cancers Article Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group Candice Hober 1,†,‡, Lisa Fredeau 2,†,‡, Anne Pham-Ledard 3,‡, Marouane Boubaya 2,‡, Florian Herms 4,‡, Philippe Celerier 5,‡, François Aubin 6,‡, Nathalie Beneton 7,‡, Monica Dinulescu 8,‡, Arnaud Jannic 9,‡, Nicolas Meyer 10,11,‡, Anne-Bénédicte Duval-Modeste 12,‡, Laure Cesaire 13,‡, Ève-Marie Neidhardt 14,‡, Élodie Archier 15,‡, Brigitte Dréno 16,17,18,‡, Candice Lesage 19,‡, Clémence Berthin 20,‡, Nora Kramkimel 21,‡, Florent Grange 22,23,‡,, Julie de Quatrebarbes 24,‡, Pierre-Emmanuel Stoebner 25,26,‡, Nicolas Poulalhon 27,‡, Jean-Philippe Arnault 28,‡, Safia Abed 29,‡, Bertille Bonniaud 30,‡, Sophie Darras 31,‡, Valentine Heidelberger 32,‡, Suzanne Devaux 33,‡, Marie Moncourier 34,‡, Laurent Misery 35,‡ , Sandrine Mansard 36,‡, Maxime Etienne 37,‡, Florence Brunet-Possenti 38,‡ , Caroline Jacobzone 39,‡, Romain Lesbazeilles 40,41,‡,§, François Skowron 23,‡,k, Julia Sanchez 22,‡,¶ , Stéphanie Catala 42,‡, Mahtab Samimi 43,44,‡, Youssef Tazi 45,‡, Dominique Spaeth 46,‡, Caroline Gaudy-Marqueste 47,‡, Olivier Collard 48,‡, Raoul Triller 49,‡, Marc Pracht 50,‡ , Marc Dumas 51,‡, Lucie Peuvrel 52,‡ , Pierre Combe 53,‡ , Olivier Lauche 54,‡, Pierre Guillet 55,‡, Yves Reguerre 56,‡, Ingrid Kupfer-Bessaguet 41,‡, David Solub 57,‡, Amélie Schoeffler 58,‡, Christophe Bedane 30,59,‡, Gaëlle Quéreux 16,17,18,‡, Sophie Dalac 30,‡, Laurent Mortier 1,60,‡ and Ève Maubec 2,61,62,*,‡ 1 Centre -

Cutaneous Manifestations of Diabetes Mellitus: Clinical Meaning Florencia Galdeano1, Sandra Zaccaria2, Viviana Parra3, María Elvira Giannini4, Susana Salomón5
ORIGINAL ARTICLES 117 Cutaneous manifestations of diabetes mellitus: clinical meaning Florencia Galdeano1, Sandra Zaccaria2, Viviana Parra3, María Elvira Giannini4, Susana Salomón5 ABSTRACT Background. Diabetes mellitus as a chronic disease it is associated to multiple complications. As Keywords: other organs, skin is affected showing different manifestations. Objetive. The aim of this study was to determinate the different types of skin lesions in diabetes diabetes mellitus, mellitus and to analyze their prevalence and their clinical correlation with other systemic cutaneous complications of this disease. Finally, to make a comparative statistical study between patients with manifestations. and without diabetic dermopathy (DD). Methods. We included 125 diabetics in a six month period. They were only Internal Medicine Department’s in-patients. Design. We made a protocoled, transversal, descriptive and observable study. Results. The 88% had type 2 diabetes mellitus. 57% were female and the mean age was 58.9 years. Eighty-eight patients had DM chronic complications: 35% DD, 32% nephropathy, 26% retinopathy, 41% neuropathy and 15% macroangiopathy. The average of cutaneous lesions per patient was 4,4. The 90.4% had cutaneous manifestations, being the most frequent: xeroderma (69%), dermatophytosis (52.8%), peripheral hypotrichia (39%), DD (35%). After doing a comparative analyze between patients with and without DD, we found, in the fi rst group more frequently (p<0,05) the followings variants: male sex, over 50 years old, retinopathy, sensitive neuropathy, macroangiopathy, diabetic foot and tinea pedis. Conclusions. The cutaneous lesions more frequently found in diabetic patients were xeroderma, tinea pedis, onychomycosis, peripheral hypotrichia and DD. As we found a statistic signifi cant association between DD and some systemic complications of DM, we propose to use this cutaneous sign as a marker of internal complications (Dermatol Argent 2010;16(2):117-121). -

Xeroderma Pigmentosum with Ocular Squamous Cell Carcinoma-A Case Report
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Case Report Xeroderma Pigmentosum with Ocular Squamous Cell Carcinoma-A Case Report Farida Tabri Dermatology and Venereology Department, Medical Faculty of Hasanuddin University, Wahidin Sudirohusodo Hospital, Makassar, Indonesia ABSTRACT Xeroderma pigmentosum (XP) is a rare autosomal recessive genodermatoses characterized by increased cellular photosensitivity to ultraviolet radiation and early onset of skin malignancy. XP may occur in any race or gender. This disease is usually acquired in infancy or childhood and is usually detected at the age of 1-2 years when sun exposure commonly starts. Clinically, the first abnormality found is extreme sensitivity to sunlight, abnormal lentigo can be found in areas exposed to sunlight. This is followed by an increase or decrease in pigmentation, aging of the skin and an increase in the incidence of skin cancer. A clinical diagnosis is confirmed by a cellular test to repair damaged DNA. We report a case of XP in a 2-year-old boy with clinical symptoms of lentiginous spots in the sun- exposed area, accompanied by Squamous Cell Carcinoma (SCC) in the eye based on histopathological examination. Multidisciplinary approach with the ophthalmology department was done to remove the SCC. The patient was given education about lifetime sun protection measurements and the importance of regular follow-up to detect the occurrence of new lesions or worsening of preexisting lesions. Key words: Xeroderma pigmentosum (XP), Ocular squamous cell carcinoma, genodermatoses INTRODUCTION replication of damaged DNA in leading Xeroderma pigmentosum (XP) is a strands. [2-3] rare autosomal recessive genodermatoses XP may occur in any race or gender. -
THE PROBLEM of ECZEMA in INFANCY and CHILDHOOD by REGINALD T
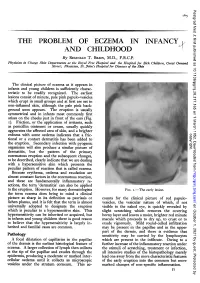
Postgrad Med J: first published as 10.1136/pgmj.28.317.165 on 1 March 1952. Downloaded from r65 THE PROBLEM OF ECZEMA IN INFANCY AND CHILDHOOD By REGINALD T. BRAIN, M.D., F.R.C.P. Physician in Charge Skin Departments at the Royal Free Hospital and the Hospital for Sick Children, Great Ormond Street; Physician, St. John's Hospital for Diseases of the Skin The clinical picture of eczema as it appears in infants and young children is sufficiently charac- teristic to be readily recognized. The earliest lesions consist of minute, pale pink papulo-vesicles which erupt in small groups and at first are set in non-inflamed skin, although the pale pink back- ground soon appears. The eruption is usually symmetrical and in infants most commonly first arises on the cheeks just in front of the ears (Fig. i). Friction, or the application of irritants, such as penicillin ointment or cream, usually quickly the affected area of and a aggravates skin, brighter copyright. redness with some oedema indicates that a fric- tional or a contact dermatitis has been added to the eruption. Secondary infection with pyogenic organisms will also produce a similar picture of dermatitis, but the pattern of the primary eczematous eruption and the subsequent changes, to be described, clearly indicate that we are dealing with a hypersensitive skin which presents the of reaction that is called eczema. peculiar pattern http://pmj.bmj.com/ Because erythema, oedema and exudation are almost constant factors in the eczematous reaction, and these are fundamentally inflammatory re- actions, the term 'dermatitis' can also be applied to the eruption.