An Arid Zone Awash with Diversity: Patterns in the Distribution of Aquatic Invertebrates in the Pilbara Region of Western Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Documentary Records Locating Aboriginal People on Mulga Downs Pre 1922 Identified Marriage / Death Certificates (From W.A
Documentary Records locating Aboriginal people on Mulga Downs pre 1922 Identified Marriage / Death certificates (from W.A. Registry Services) - Death Certificate Registration 18/1955, Roebourne district - Tommy Tucker (stockman) male aged 73 born (c.1882) at Mulga Downs Station, son of unknown parents, no marriage details recorded, no children recorded, deceased 10/12/1955 at Roebourne district hospital, buried 10/12/1955 in native portion Roebourne cemetery, last resident at Mulga Downs station prior to death. Note: witnesses were Tumbler and F. Hick - Death Certificate Registration 20/1956, Roebourne district - Banjo (indigent) male age 75 born (c1881) at Mulga Downs, child of Peter (occupation unknown) and Polly spouse not recorded, children Ada (age unknown), date of death 08/12/1956, deceased at Mulga Downs Station, buried at Native Portion Mulga Downs cemetery, last resident at Mulga Downs Station, Wittenoom prior to death. This could possibly be a record of Banjo shown by Palmer to be a partner of ivy Tucker @ Naijong : p. 26 Ivy Tucker shown as mother of Harold (father Harold Mayor, Euro, partner of Daphne Jones); Selina (father Gayuna), Blanche Tucker (father Gayuna), Gertie (father Gayuna), Eric Cosmo (father Spiro Cosmos, Greek); Selina (father Gayuna, partner of Pat Long), Blanche Tucker (father Gayuna), Gertie (father Gayuna, partner of Ginger Parker), John Douglas MacArthur (father McArthur, Euro); Eustace (partner of Dulcie Tumbler) and Ivy also partner of Spider (Ngarla man, no children), Banjo (no children) and Hickey Bung. - Death Certificate Registration 18/1956, Roebourne district - Sally Dundy (indigent native) female age 80 born (c1876) at Mulga Downs, Wittenoom, child of unknown parents, spouse not recorded, no children recorded, date of death 19/10/1956, deceased at Mulga Downs Station, buried at Native Portion Mulga Downs cemetery, last resident at Mulga Downs station, Wittenoom prior to death. -

The Cyclone As Trope of Apocalypse and Place in Queensland Literature
ResearchOnline@JCU This file is part of the following work: Spicer, Chrystopher J. (2018) The cyclone written into our place: the cyclone as trope of apocalypse and place in Queensland literature. PhD Thesis, James Cook University. Access to this file is available from: https://doi.org/10.25903/7pjw%2D9y76 Copyright © 2018 Chrystopher J. Spicer. The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owners of any third party copyright material included in this document. If you believe that this is not the case, please email [email protected] The Cyclone Written Into Our Place The cyclone as trope of apocalypse and place in Queensland literature Thesis submitted by Chrystopher J Spicer M.A. July, 2018 For the degree of Doctor of Philosophy College of Arts, Society and Education James Cook University ii Acknowledgements of the Contribution of Others I would like to thank a number of people for their help and encouragement during this research project. Firstly, I would like to thank my wife Marcella whose constant belief that I could accomplish this project, while she was learning to live with her own personal trauma at the same time, encouraged me to persevere with this thesis project when the tide of my own faith would ebb. I could not have come this far without her faith in me and her determination to journey with me on this path. I would also like to thank my supervisors, Professors Stephen Torre and Richard Landsdown, for their valuable support, constructive criticism and suggestions during the course of our work together. -

Research Priorities for the Northern Quoll (Dasyurus Hallucatus) in the Pilbara Region of Western Australia
CSIRO PUBLISHING Australian Mammalogy Review http://dx.doi.org/10.1071/AM15005 Research priorities for the northern quoll (Dasyurus hallucatus) in the Pilbara region of Western Australia Viki A. Cramer A, Judy Dunlop A, Rob Davis B, Ryan Ellis C, Belinda Barnett D, Annette Cook A, Keith Morris A and Stephen van Leeuwen A,E AScience and Conservation Division, Department of Parks and Wildlife, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia. BSchool of Natural Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, WA 6027, Australia. CDepartment of Terrestrial Zoology, Western Australian Museum, 49 Kew Street, Welshpool, WA 6106, Australia. DEnvironment Department, BHP Billiton Iron Ore, 125 St Georges Terrace, Perth, WA 6000, Australia. ECorresponding author. Email: [email protected] Abstract. The Pilbara population of the northern quoll (Dasyurus hallucatus) has been seldom studied, and the impacts of threats such as altered fire regimes, total grazing pressure, predation and mining and infrastructure development are not well understood. While the Pilbara was once thought likely to provide refuge for northern quolls from the poisonous cane toad (Rhinella marina), recent modelling suggests that cane toads will invade the region. The environmental approvals process for mining development in the Pilbara has generated considerable offset funds that are to be directed towards research on the northern quoll. In an effort to identify future research priorities for this species in the Pilbara through a collaborative -

Module One Understanding Tropical Cyclone
Module One Understanding Tropical Cyclone About this Module Students take part in a collection of activities, designed to give them a basic understanding of the impact a tropical cyclone has on Western Australian coastal areas. They discover when, where and how often tropical cyclones occur in Western Australia and the type of weather associated with tropical cyclones. Background Information A tropical cyclone is a natural hazard that brings with it damaging winds, heavy rainfall, flooding and storm surge. Cyclone activity produces strong onshore winds and flooding rains, which increases the threat of storm surge. Sea levels can rise rapidly as a tropical cyclone makes landfall resulting in storm surge impacting low-lying coastal areas during and after a cyclone. The heavy rainfall associated with cyclones can result in flash and broad-scale flooding. Communities who do not experience a direct tropical cyclone impact can still experience widespread flooding. Western Australia experiences tropical cyclones every year, normally affecting the coast in the Kimberley, Pilbara and Midwest Gascoyne regions. Cyclones can also affect inland parts of northern WA and can travel down the coast and threaten communities further south. The coastline between Exmouth and Broome has the highest incidence of cyclones anywhere in Australia. This area is commonly referred to as ‘Cyclone Alley’. On average, five tropical cyclones are expected to form in waters off the northwest coast of WA each season between November and April. Two of these cyclones are likely to cross the northwest coast, with one crossing as a severe tropical cyclone. The average life cycle for most cyclones is one week. -

The Riparian Flora and Plant Communities of the Pilbara Region Of
DOI: 10.18195/issn.0313-122x.78(2).2015.485-513 Records of the Western Australian Museum, Supplement 78: 485–513 (2015). The riparian fl ora and plant communities of the Pilbara region of Western Australia M.N. Lyons Department of Parks and Wildlife, Science and Conservation Division, Kieran McNamara Conservation Science Centre, Locked Bag 104, Bentley Delivery Centre, Western Australia 6983, Australia. Email: [email protected] Abstract – A survey of riparian fl ora and plant communities was undertaken at 98 wetlands and rivers in the Pilbara region of Western Australia. Sampling was quadrat-based, with fl oristics, surface soils and wetland attributes recorded. Selected sites captured the full range of Pilbara wetland types including springs, river pools, claypans, salt marshes and rock pools. A total of 455 taxa was recorded from the survey sites, representing ca. 25% of the known fl ora of the Pilbara bioregion. The fl ora is dominated by taxa with Eremaean and tropical affi nities, with only six taxa endemic in the region. Of recorded taxa known from four or fewer bioregions, most are shared with the adjacent Carnarvon and Gascoyne bioregions rather than the adjoining internally draining deserts. Sixteen taxa of conservation signifi cance were documented, with claypans, the Fortescue Marsh, and Millstream and Karijini National Park sites dominating occurrences of rare species. Eight major groups were defi ned by classifying wetlands in terms of species presence/absence data. Floristic patterning was strongly aligned with the major wetland types (geomorphic/hydrological) used in the primary sampling stratifi cation. A combination of wetland morphology/hydrological setting, site edaphic attributes and distance to the coast were dominant variables related to riparian fl oristic composition. -
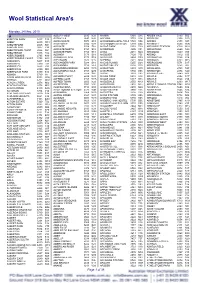
Wool Statistical Area's
Wool Statistical Area's Monday, 24 May, 2010 A ALBURY WEST 2640 N28 ANAMA 5464 S15 ARDEN VALE 5433 S05 ABBETON PARK 5417 S15 ALDAVILLA 2440 N42 ANCONA 3715 V14 ARDGLEN 2338 N20 ABBEY 6280 W18 ALDERSGATE 5070 S18 ANDAMOOKA OPALFIELDS5722 S04 ARDING 2358 N03 ABBOTSFORD 2046 N21 ALDERSYDE 6306 W11 ANDAMOOKA STATION 5720 S04 ARDINGLY 6630 W06 ABBOTSFORD 3067 V30 ALDGATE 5154 S18 ANDAS PARK 5353 S19 ARDJORIE STATION 6728 W01 ABBOTSFORD POINT 2046 N21 ALDGATE NORTH 5154 S18 ANDERSON 3995 V31 ARDLETHAN 2665 N29 ABBOTSHAM 7315 T02 ALDGATE PARK 5154 S18 ANDO 2631 N24 ARDMONA 3629 V09 ABERCROMBIE 2795 N19 ALDINGA 5173 S18 ANDOVER 7120 T05 ARDNO 3312 V20 ABERCROMBIE CAVES 2795 N19 ALDINGA BEACH 5173 S18 ANDREWS 5454 S09 ARDONACHIE 3286 V24 ABERDEEN 5417 S15 ALECTOWN 2870 N15 ANEMBO 2621 N24 ARDROSS 6153 W15 ABERDEEN 7310 T02 ALEXANDER PARK 5039 S18 ANGAS PLAINS 5255 S20 ARDROSSAN 5571 S17 ABERFELDY 3825 V33 ALEXANDRA 3714 V14 ANGAS VALLEY 5238 S25 AREEGRA 3480 V02 ABERFOYLE 2350 N03 ALEXANDRA BRIDGE 6288 W18 ANGASTON 5353 S19 ARGALONG 2720 N27 ABERFOYLE PARK 5159 S18 ALEXANDRA HILLS 4161 Q30 ANGEPENA 5732 S05 ARGENTON 2284 N20 ABINGA 5710 18 ALFORD 5554 S16 ANGIP 3393 V02 ARGENTS HILL 2449 N01 ABROLHOS ISLANDS 6532 W06 ALFORDS POINT 2234 N21 ANGLE PARK 5010 S18 ARGYLE 2852 N17 ABYDOS 6721 W02 ALFRED COVE 6154 W15 ANGLE VALE 5117 S18 ARGYLE 3523 V15 ACACIA CREEK 2476 N02 ALFRED TOWN 2650 N29 ANGLEDALE 2550 N43 ARGYLE 6239 W17 ACACIA PLATEAU 2476 N02 ALFREDTON 3350 V26 ANGLEDOOL 2832 N12 ARGYLE DOWNS STATION6743 W01 ACACIA RIDGE 4110 Q30 ALGEBUCKINA -

Looking West: a Guide to Aboriginal Records in Western Australia
A Guide to Aboriginal Records in Western Australia The Records Taskforce of Western Australia ¨ ARTIST Jeanette Garlett Jeanette is a Nyungar Aboriginal woman. She was removed from her family at a young age and was in Mogumber Mission from 1956 to 1968, where she attended the Mogumber Mission School and Moora Junior High School. Jeanette later moved to Queensland and gained an Associate Diploma of Arts from the Townsville College of TAFE, majoring in screen printing batik. From 1991 to present day, Jeanette has had 10 major exhibitions and has been awarded four commissions Australia-wide. Jeanette was the recipient of the Dick Pascoe Memorial Shield. Bill Hayden was presented with one of her paintings on a Vice Regal tour of Queensland. In 1993 several of her paintings were sent to Iwaki in Japan (sister city of Townsville in Japan). A recent major commission was to create a mural for the City of Armadale (working with Elders and students from the community) to depict the life of Aboriginal Elders from 1950 to 1980. Jeanette is currently commissioned by the Mundaring Arts Centre to work with students from local schools to design and paint bus shelters — the established theme is the four seasons. Through her art, Jeanette assists Aboriginal women involved in domestic and traumatic situations, to express their feelings in order to commence their journey of healing. Jeanette currently lives in Northam with her family and is actively working as an artist and art therapist in that region. Jeanette also lectures at the O’Connor College of TAFE. Her dream is to have her work acknowledged and respected by her peers and the community. -

Annual Report to the Pastoral Lands Board of Western Australia 2003–04
Annual Report to the Pastoral Lands Board of Western Australia 2003/2004 Financial Year A report prepared for the Pastoral Lands Board of Western Australia by the Department of Agriculture October 2004 Compiled by Rod Williams and Philip Thomas with contributions from rangeland staff CONTENTS PREFACE 3 EXECUTIVE SUMMARY 4 INTRODUCTION 6 PASTORAL LEASES 6 THE DIVERSITY OF THE RANGELANDS 6 1. ENVIRONMENTAL FACTORS 1.1 WARMS 7 1.2 Pastoral lease inspections 9 1.3 Rangelands resource surveys 13 1.4 Seasonal conditions 16 2. ECONOMIC ISSUES 2.1 Economic data 21 2.2 Trends in stock numbers compared to potential carrying capacities 29 2.3 Trends in cattle turnoff and wool cuts per head 31 2.4 Pastoral lease property market and rentals 31 3. SOCIAL INDICATORS 3.1 Kimberley 33 3.2 Pilbara 35 3.3 Gascoyne 38 3.4 Mid-West 40 3.5 Goldfields-Esperance (Nullarbor) 41 APPENDIX Graphical representation of rangeland statistics 43 2 Preface The Department of Agriculture has prepared this Annual Report for the Pastoral Lands Board of Western Australia for the financial year 2003 – 2004. This is the second report incorporating this framework and provides expanded information on issues and trends for a number of indicators of importance to Western Australia’s rangelands. The Department of Agriculture’s services to the Board are outlined and environmental indicators based on scientific analysis of range condition trend are presented at the regional and property scales. Seasonal conditions at the state level with more specific information at the regional level and information relating to the assistance packages available for those leases located in the area covered by the Exceptional Circumstances for 16 Shires in the regional areas of Pilbara; Gascoyne; Murchison; and Goldfields is included. -

Pilbara 2 (PIL2 – Fortescue Plains Subregion)
Pilbara 2 Pilbara 2 (PIL2 – Fortescue Plains subregion) PETER KENDRICK OCTOBER 2001 Subregional description and biodiversity of the aquifer, where either the Fortescue River or associated streams have eroded into the water- values carrying calcrete. The aquifer is known to contain a stygofauna. Little is known of this fauna, due to lack Description and area of survey. • Alluvial plains and river frontage. Extensive salt marsh, Fortescue Marsh: An extensive, episodically mulga-bunch grass, and short grass communities on inundated samphire marsh, approximately 100 km alluvial plains in the east. Deeply incised gorge systems in long and 10 km wide. Constricted at the western the western (lower) part of the drainage. River gum (downstream) end by the Goodiadarrie Hills, it is woodlands fringe the drainage lines. Northern limit of possible that the upper Fortescue is prevented from Mulga (Acacia aneura). An extensive calcrete aquifer flowing through into the lower Fortescue drainage (originating within a palaeo-drainage valley) feeds except in extreme rainfall events. These hills numerous permanent springs in the central Fortescue, effectively separate the Fortescue into two separate supporting large permanent wetlands with extensive drainages. The Fortescue Marsh represents the stands of river gum and cadjeput Melaleuca woodlands. terminus for the upper Fortescue. Episodically Climatic conditions are semi desert tropical, with average supports immense water-bird breeding. rainfall of 300 mm, falling mainly in summer cyclonic events. Drainage occurs to the north-west. Subregional Short Range Endemics area is 2,041,914ha. Generally very little is known about short range endemic invertebrates in the Pilbara. Dominant land use Rare Vertebrates (see Appendix B, key b) Includes: Bilby (Macrotis lagotis) and Orange Leaf-nosed Bat (Rhinonicteris aurantius). -

Birds of the Pilbara Region, Western Australia, 1967-1972 by TOM FLETCHER, 7 Montgomery Court, Kilsyth, Victoria, 3137
AUSTRALIAN 220 BIRD WATCHER Birds of the Pilbara Region, Western Australia, 1967-1972 By TOM FLETCHER, 7 Montgomery Court, Kilsyth, Victoria, 3137. Introduction In 1967, after just returning from New Guinea, I was contracted to work on the initial studies and design for the Mt. Newman Iron Ore Project at Port Hedland, North-West Australia. I mention New Guinea in the above paragraph only to make a com parison with North-West Australia, for there are absolutely no similarities whatsoever between the two localities. The contrast is so extreme that on arriving in Port Hedland I was convinced I would not be there the following week at the same time. Nevertheless it was six and a half years later, and then only for the sake of my sanity, that I vacated the North-West Pilbara at the conclusion of a most rewarding and satisfying experience. During my years in the Pilbara I travelled extensively and continuously throughout the area radiating from Port Hedland and mainly bounded by Roebourne to the west-south-west, Wittenoom and Mt. Newman to the south, Marble Bar and Nullagine to the south-east and De Grey Station to the north-east. This area is primarily mineral rich granitic spinifex plains with very little tall vegetation apart from along the normally dry river courses with very occasional permanent water holes and of course mill bores. Rising from the plains are several most impressive ranges such as the Hamersley, Chichester and Marble Bar. The coastal areas are mainly sandy beaches and tidal mud flats with the tide being up to 10 metres. -

Official/Settler Names Index
Official/Settler Location Station/Mission/Reserve File Number Abraham, Mr 313/1904 Abraham, Mr J.S. West Perth 421/1906 Abraham, Percy 557/1903 Abraham, Percy Nor West 421/1906 Adam, J.P. York 319/1901 Adam, John (RM Northam) Northam 588/1899 Adam, John (RM Northam) Northam 471/1898 Adam, Mr 387/1898 Adam, W.H. (RM Katanning) Katanning 15/1898 Adam, W.K. (RM Katanning) Katanning 185/1899 Adam, W.K. (RM Katanning) Katanning 441/1898 Adam, W.K. (RM Katanning) Katanning 342/1900 Adam, W.K. (RM Katanning) Katanning 330/1898 Adam, W.K. (RM Katanning) Katanning 150/1899 Adam, W.K. (RM Kattanning) Katanning 353/1898 Adams (Const. No. 202) Dongarra 666/1906 Adams, Arthur R. (Actg. RM Onslow) Onslow 612/1907 Adams, Arthur R. (RM Derby) Derby Unnumbered/1908 Adams, Arthur R. (RM Derby) Derby 957/1908 Adams, Dr (DMO) Derby 799/1908 Adams, Jane Mangowine 279/1900 Adams, Jane 665/1898 Adams, Jane Mangonine 93/1905 Adams, Mr Derby 797/1908 Adams, Mr Onslow 349/1908 Adams, Mr (RM Derby) Derby 409B/1908 Adams, Mr J. Mangowine 279/1901 Adams, Mrs Yanajin Station Yanajin Station 1098/1906 Adams, Mrs Shark Bay 11/1905 Adams, W.J. Dongarra 328/1908 Adams, W.J. (Const. No. 202) Dongarra Unnumbered/1908 Adams, W.J. (Const. No. 202) 442/1901 Adcock, Mr C.J. Derby 616/1902 Adcock, Mrs Derby 762/1907 Adcock, Mrs (nee Thompson) Derby 616/1902 Ah Chew (m-Malay) Quanborn Station (?) Quanborn Station (?) 835/1908 Ahern, H.N. Twenty Mile Sandy 647/1902 Aikman, Andy Cook Creek 284/1908 Aitchison, J. -

Worksheets Cyclone and Flood
Worksheets Cyclone and Flood Module One: Understanding Tropical Cyclones Tropical Cyclone (TC) Facts information sheet Cyclops – a giant with only one eye information sheet Tropical Cyclone Fay worksheet Module Two: What’s In a Name? Tropical Cyclone Damage – information sheet Tropical Cyclone Damage – worksheet Module Three: Understanding Flood Types of Flood – worksheet Types of Flood – answer sheet Town Engineer for a Day! – information sheet Before, During and After a Flood – worksheet Before, During and After a Flood – answer sheet Module Four: Staying Safe in Cyclone and Flood Risky Behaviours – Turn Around, Don’t Drown – information sheet What could a 3 metre water level mean to your classroom – worksheet Module Five: Have a Plan - Cyclone and Flood Your School’s Emergency Plan – information sheet Look at your School’s Emergency Plan – worksheet Emergency Contact Numbers – worksheet Emergency Kit and Relocation Kit – worksheet How can you PREPARE for cyclone and/or flood emergencies? – worksheet Module Six: Responding to Cyclone and Flood DFES Cyclone Smart Community Alerts – worksheet From Rain to Warning – information sheet Tropical Cyclone (TC) Facts (Cut along dotted lines) _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ Tropical cyclones are storms that form over warm tropical waters and have gale force winds near their centre. Gale force winds are winds moving at 63 kilometres per hour (km/h) or greater. Above the equator (in the northern hemisphere), cyclones spin in an anti-clockwise direction and in the southern hemisphere they spin in a clockwise direction.