Studies on the Hydrobiology and Pollution of the Vemband Lake And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with Financial Assistance from the World Bank
KERALA SOLID WASTE MANAGEMENT Public Disclosure Authorized PROJECT (KSWMP) INTRODUCTION AND STRATEGIC ENVIROMENTAL ASSESSMENT OF WASTE Public Disclosure Authorized MANAGEMENT SECTOR IN KERALA VOLUME I JUNE 2020 Public Disclosure Authorized Prepared by SUCHITWA MISSION Public Disclosure Authorized GOVERNMENT OF KERALA Contents 1 This is the STRATEGIC ENVIRONMENTAL ASSESSMENT OF WASTE MANAGEMENT SECTOR IN KERALA AND ENVIRONMENTAL AND SOCIAL MANAGEMENT FRAMEWORK for the KERALA SOLID WASTE MANAGEMENT PROJECT (KSWMP) with financial assistance from the World Bank. This is hereby disclosed for comments/suggestions of the public/stakeholders. Send your comments/suggestions to SUCHITWA MISSION, Swaraj Bhavan, Base Floor (-1), Nanthancodu, Kowdiar, Thiruvananthapuram-695003, Kerala, India or email: [email protected] Contents 2 Table of Contents CHAPTER 1. INTRODUCTION TO THE PROJECT .................................................. 1 1.1 Program Description ................................................................................. 1 1.1.1 Proposed Project Components ..................................................................... 1 1.1.2 Environmental Characteristics of the Project Location............................... 2 1.2 Need for an Environmental Management Framework ........................... 3 1.3 Overview of the Environmental Assessment and Framework ............. 3 1.3.1 Purpose of the SEA and ESMF ...................................................................... 3 1.3.2 The ESMF process ........................................................................................ -

Report on Visit to Vembanad Kol, Kerala, a Wetland Included Under
Report on Visit to Vembanad Kol, Kerala, a wetland included under the National Wetland Conservation and Management Programme of the Ministry of Environment and Forests. 1. Context To enable Half Yearly Performance Review of the programmes of the Ministry of Environment & Forests, the Planning Commission, Government of India, on 13th June 2008 constituted an Expert Team (Appendix-1) to visit three wetlands viz. Wular Lake in J&K, Chilika Lake in Orissa and Vembanad Kol in Kerala, for assessing the status of implementation of the National Wetland Conservation and Management Programme (NWCMP). 2. Visit itinerary The Team comprising Dr.(Mrs.) Indrani Chandrasekharan, Advisor(E&F), Planning Commission, Dr. T. Balasubramanian, Director, CAS in Marine Biology, Annamalai University and Dr. V. Sampath, Ex-Advisor, MoES and UNDP Sr. National Consultant, visited Vembanad lake and held discussions at the Vembanad Lake and Alleppey on 30 June and 1st July 2008. Details of presentations and discussions held on 1st July 2008 are at Appendix-2. 3. The Vembanad Lake Kerala has a continuous chain of lagoons or backwaters along its coastal region. These water bodies are fed by rivers and drain into the Lakshadweep Sea through small openings in the sandbars called ‘azhi’, if permanent or ‘pozhi’, if temporary. The Vembanad wetland system and its associated drainage basins lie in the humid tropical region between 09˚00’ -10˚40’N and 76˚00’-77˚30’E. It is unique in terms of physiography, geology, climate, hydrology, land use and flora and fauna. The rivers are generally short, steep, fast flowing and monsoon fed. -

A CONCISE REPORT on BIODIVERSITY LOSS DUE to 2018 FLOOD in KERALA (Impact Assessment Conducted by Kerala State Biodiversity Board)
1 A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) Editors Dr. S.C. Joshi IFS (Rtd.), Dr. V. Balakrishnan, Dr. N. Preetha Editorial Board Dr. K. Satheeshkumar Sri. K.V. Govindan Dr. K.T. Chandramohanan Dr. T.S. Swapna Sri. A.K. Dharni IFS © Kerala State Biodiversity Board 2020 All rights reserved. No part of this book may be reproduced, stored in a retrieval system, tramsmitted in any form or by any means graphics, electronic, mechanical or otherwise, without the prior writted permission of the publisher. Published By Member Secretary Kerala State Biodiversity Board ISBN: 978-81-934231-3-4 Design and Layout Dr. Baijulal B A CONCISE REPORT ON BIODIVERSITY LOSS DUE TO 2018 FLOOD IN KERALA (Impact assessment conducted by Kerala State Biodiversity Board) EdItorS Dr. S.C. Joshi IFS (Rtd.) Dr. V. Balakrishnan Dr. N. Preetha Kerala State Biodiversity Board No.30 (3)/Press/CMO/2020. 06th January, 2020. MESSAGE The Kerala State Biodiversity Board in association with the Biodiversity Management Committees - which exist in all Panchayats, Municipalities and Corporations in the State - had conducted a rapid Impact Assessment of floods and landslides on the State’s biodiversity, following the natural disaster of 2018. This assessment has laid the foundation for a recovery and ecosystem based rejuvenation process at the local level. Subsequently, as a follow up, Universities and R&D institutions have conducted 28 studies on areas requiring attention, with an emphasis on riverine rejuvenation. I am happy to note that a compilation of the key outcomes are being published. -
List of Lacs with Local Body Segments (PDF
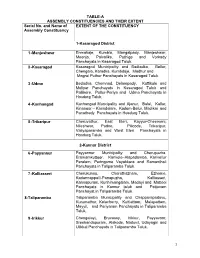
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -

Ground Water Information Booklet of Alappuzha District
TECHNICAL REPORTS: SERIES ‘D’ CONSERVE WATER – SAVE LIFE भारत सरकार GOVERNMENT OF INDIA जल संसाधन मंत्रालय MINISTRY OF WATER RESOURCES कᴂ द्रीय भजू ल बो셍 ड CENTRAL GROUND WATER BOARD केरल क्षेत्र KERALA REGION भूजल सूचना पुस्तिका, मलꥍपुरम स्ज쥍ला, केरल रा煍य GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA STATE तत셁वनंतपुरम Thiruvananthapuram December 2013 GOVERNMENT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA जी श्रीनाथ सहायक भूजल ववज्ञ G. Sreenath Asst Hydrogeologist KERALA REGION BHUJAL BHAVAN KEDARAM, KESAVADASAPURAM NH-IV, FARIDABAD THIRUVANANTHAPURAM – 695 004 HARYANA- 121 001 TEL: 0471-2442175 TEL: 0129-12419075 FAX: 0471-2442191 FAX: 0129-2142524 GROUND WATER INFORMATION BOOKLET OF MALAPPURAM DISTRICT, KERALA TABLE OF CONTENTS DISTRICT AT A GLANCE 1.0 INTRODUCTION ..................................................................................................... 1 2.0 CLIMATE AND RAINFALL ................................................................................... 3 3.0 GEOMORPHOLOGY AND SOIL TYPES .............................................................. 4 4.0 GROUNDWATER SCENARIO ............................................................................... 5 5.0 GROUNDWATER MANAGEMENT STRATEGY .............................................. 11 6.0 GROUNDWATER RELATED ISaSUES AND PROBLEMS ............................... 14 7.0 AWARENESS AND TRAINING ACTIVITY ....................................................... 14 -

Vembanad Lake Spreads Vol 3:5 Over an Area of 2033 Square Kilometres Covering Three Districts
Popular Article Journal Home: www.bioticainternational.com Article: RT564 How to cite this article? Biotica Das et al., 2021. Lacustrine Fisheries of Kerala. Biotica Research [Research Today 3(5): 285-289. [ Today Abstract erala is bequeathed with inland lakes and wetlands of 285 international and national importance. Vembanad Lake spreads Vol 3:5 over an area of 2033 square kilometres covering three districts. 289 TheK wetland is an ecologically sensitive habitat, famous as a Ramsar 2021 site and a critically vulnerable area. Anthropogenic activities such as illegal fishing gears, fishery aggregation devices, land reclamation, Lacustrine Fisheries of manmade interferences, pollution and natural influences of lake have led to deterioration of natural habitat as well as became a threat to Kerala aquatic diversity. Therefore, taking ameliorative strategies such as 1 policy development, strict implementation of laws, awareness and Vandana Gokul Das , Thankam Theresa conservative programmes as well as initiating mitigation strategies Paul1*, Albin Albert C.1, S. Manoharan1, like CRPS, mangrove restoration programmes, analysing the livelihood 1 2 status of fishers of the lake pertains to relieving the adverse impacts Deepa Sudeeshan and B. K. Das and enhances health status of the lake. 1ICAR- Central Inland Fisheries Research Institute, Kochi, Kerala (682 024), India Introduction 2ICAR- Central Inland Fisheries Research Institute, ndia is bestowed with diverse array of water resources Barrackpore, West Bengal (700 120), India and lakes are one such aquatic system widely recognized Ifor their multiple attributes. Lakes play a significant role to mankind being a valuable natural resource. Kerala is bequeathed with many such inland lakes and wetlands of international and national importance. -

Biodiversity Status.Qxp
163 BIODIVERSITY STATUS OF FISHES INHABITING RIVERS OF KERALA (S. INDIA) WITH SPECIAL REFERENCE TO ENDEMISM, THREATS AND CONSERVATION MEASURES Kurup B.M. Radhakrishnan K.V. Manojkumar T.G. School of Industrial Fisheries, Cochin University of Science & Technology, Cochin 682 016, India E-mail: [email protected] ABSTRACT The identification of 175 freshwater fish- es from 41 west flowing and 3 east flowing river systems of Kerala were confirmed. These can be grouped under 106 ornamental and 67 food fish- es. The biodiversity status of these fishes was assessed according to IUCN criteria. The results showed that populations of the majority of fish species showed drastic reduction over the past five decades. Thirty-three fish species were found to be endemic to the rivers of Kerala. The distributions of the species were found to vary within and between the river systems and some of the species exhibited a high degree of habitat specificity. The diversity and abundance of the species generally showed an inverse relationship with altitude. The serious threats faced by the freshwater fishes of Kerala are mostly in the form of human interventions and habitat alter- ations and conservation plans for the protection and preservation of the unique and rare fish bio- diversity of Kerala are also highlighted. 164 Biodiversity status of fishes inhabiting rivers of Kerala (S.India) INTRODUCTION river. Habitat diversity was given foremost importance during selection of locations within the river system. Kerala is a land of rivers which harbour a rich The sites for habitat inventory were selected based on and diversified fish fauna characterized by many rare channel pattern, channel confinement, gradient and and endemic fish species. -

Gis Based Water Quality Analysis of Chaliyar River
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 07 Issue: 07 | July 2020 www.irjet.net p-ISSN: 2395-0072 GIS BASED WATER QUALITY ANALYSIS OF CHALIYAR RIVER Nisanth Nan¹, Revathi P² ¹M. Tech student, Department of Environmental Engineering, M-DIT Engineering College ²Assistant Professor, Department of Environmental Engineering, M-DIT Engineering College 1,2A P J Abdul Kalam Technological University, Kerala, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract: Assessment of seasonal variations in surface Thus, with GIS, it is possible to map, model, query and water quality characteristics is an essential aspect for analyze large quantities of data all held together within a evaluating water pollution due to both natural and single database anthropogenic influences on water resources. In this study, temporal variations of water quality in Chaliyar river were 2. STUDY AREA assessed and an integrated water quality map was created. Water samples from eight locations along the stretch of the The river Chaliyar is located in India. Chaliyar is the river were collected for two seasons and were analysed for fourth longest river in the state of Kerala at 169 km in physicochemical and microbiological parameters such as length. The Chaliyar is also known as Chulika River or pH, turbidity, COD, DO, iron, chloride, nitrate and total Beypore River as it nears the sea. Nilambur, Edavanna, coliforms. Variations in these properties for the two seasons Areekode, Kizhuparamba, Cheruvadi, Edavannappara, were analysed and an integrated water quality map was Mavoor, Peruvayal, Feroke and Beypore are some of the created using ArcGIS software. towns/villages situated along the banks of Chaliyar. -

Feasibility Study of Roll On-Roll Off Transport Across Vembanad Lake
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395 -0056 Volume: 04 Issue: 04 | Apr -2017 www.irjet.net p-ISSN: 2395-0072 FEASIBILITY STUDY OF ROLL ON-ROLL OFF TRANSPORT ACROSS VEMBANAD LAKE Remadevi M1, Jomy M J2, Neha Ann Jaison3, Sumayya Jamal4 1 Professor, Dept. of civil Engineering, Mar Athanasius College of Engineering, Kothamangalam, Kerala, India 2,3,4Students, Dept. of civil Engineering, Mar Athanasius College of Engineering, Kothamangalam, Kerala, India ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - Kerala, famously known as ‘God’s own country’ is In India only .4% of the domestic surface transport is blessed with backwaters in abundance. Studies prove that accounted for by IWT compared with 68% by road and 30% water transportation is the cheapest mode of transportation. by rail even though India is richly endowed with navigable However, currently only 20% of the inland waters in Kerala waterways, comprising of rivers, canals, backwaters, creeks, are used for navigation. Vembanad Lake (Vembanad Kayal) is lagoons etc. the largest lake in Kerala and also counted as one of the largest lakes in India. The Inland Water Transport in Kerala includes rivers and backwaters. This has paid a major role in the Vembanad Lake is bounded by Kottayam and Alappuzha transportation right from the old period. Coastal Shipping districts. Currently the two road networks connecting and Inland Navigation Department (CSIND), State Water Alappuzha and Kottayam traverse the perimeter of the Transport Department (SWTD), and Kerala Shipping and Vembanad Lahe. By providing a Roll on –Roll off service on Inland Navigation Corporation Ltd. (KSINC) are the agencies Vembanad Lake connecting the two districts, traffic which are responsible for the development of inland water congestion on these roads can be reduced. -
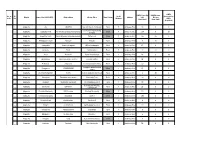
Janakeeya Hotel Updation 01.10.2020
LUNCH LUNCH Parcel By LUNCH Home No. of Sl. No Of Sponsored by District Name of the LSGD (CDS) Kitchen Name Kitchen Place Rural / Urban Initiative Unit Delivery units No. Members LSGI's (Sept 22nd ) (Sept 22nd ) (Sept 22nd) 1 Alappuzha Ala JANATHA Near CSI church, Kodukulanji Rural 5 Janakeeya Hotel 30 0 0 Coir Machine Manufacturing 2 Alappuzha Alappuzha North Ruchikoottu Janakiya Bhakshanasala Urban 4 Janakeeya Hotel 194 0 20 Company 3 Alappuzha Alappuzha South Samrudhi janakeeya bhakshanashala Pazhaveedu Urban 5 Janakeeya Hotel 107 96 0 4 Alappuzha Ambalappuzha South Patheyam Amayida Rural 5 Janakeeya Hotel 0 75 5 5 Alappuzha Arattupuzha Hanna catering unit JMS hall,arattupuzha Rural 6 Janakeeya Hotel 67 0 0 6 Alappuzha Arookutty Ruchi Kombanamuri Rural 5 Janakeeya Hotel 39 44 0 Alappuzha Aroor Navaruchi Vyasa charitable trust Rural 5 Janakeeya Hotel 28 0 58 7 Alappuzha Bharanikavu Sasneham Janakeeya Hotel Koyickal chantha Rural 5 Janakeeya Hotel 95 0 0 8 Alappuzha Budhanoor sampoorna mooshari parampil building Rural 5 Janakeeya Hotel 60 0 0 9 chengannur market building Alappuzha Chenganoor SRAMADANAM Urban 5 Janakeeya Hotel 65 0 0 10 complex Alappuzha Chennam Pallippuram Friends Chennam pallipuram panchayath Rural 3 Janakeeya Hotel 3 50 0 11 Alappuzha Chennithala Bhakshana sree canteen Chennithala Town Rural 4 Janakeeya Hotel 30 0 0 12 Alappuzha Cheppad Sreebhadra catering unit Choondupalaka junction Rural 3 Janakeeya Hotel 79 0 0 13 Near GOLDEN PALACE Alappuzha Cheriyanad DARSANA Rural 5 Janakeeya Hotel 25 0 0 14 AUDITORIUM Alappuzha -

CDS) Members (December 20) (December 20) (December 20)
LUNCH Parcel By LUNCH Home LUNCH Sponsored Sl. Name of the LSGD No Of District Kitchen Name Kitchen Place Rural / Urban Initiative Unit Delivery by LSGI's No. (CDS) Members (December 20) (December 20) (December 20) 1 Alappuzha Ala JANATHA Near CSI church, Kodukulanji Rural 5 Janakeeya Hotel 0 0 0 2 Alappuzha Alappuzha North Ruchikoottu Janakiya Bhakshanasala Coir Machine Manufacturing Company Urban 4 Janakeeya Hotel 50 0 0 3 Alappuzha Alappuzha South Samrudhi janakeeya bhakshanashala Pazhaveedu Urban 5 Janakeeya Hotel 0 0 0 4 Alappuzha Alappuzha South Community kitchen thavakkal group MCH junction Urban 5 Janakeeya Hotel 0 0 0 5 Alappuzha Ambalppuzha North Swaruma Neerkkunnam Rural 10 Janakeeya Hotel 0 0 0 6 Alappuzha Ambalappuzha South Patheyam Amayida Rural 5 Janakeeya Hotel 0 0 0 7 Alappuzha Arattupuzha Hanna catering unit JMS hall,arattupuzha Rural 6 Janakeeya Hotel 0 0 0 8 Alappuzha Arookutty Ruchi Kombanamuri Rural 5 Janakeeya Hotel 0 0 0 9 Alappuzha Aroor Navaruchi Vyasa charitable trust Rural 5 Janakeeya Hotel 23 0 0 10 Alappuzha Aryad Anagha Catering Near Aryad Panchayat Rural 5 Janakeeya Hotel 0 0 0 11 Alappuzha Bharanikavu Sasneham Janakeeya Hotel Koyickal chantha Rural 5 Janakeeya Hotel 0 0 0 12 Alappuzha Budhanoor sampoorna mooshari parampil building Rural 5 Janakeeya Hotel 0 0 0 13 Alappuzha Chambakulam Jyothis Near party office Rural 4 Janakeeya Hotel 0 0 0 14 Alappuzha Chenganoor SRAMADANAM chengannur market building complex Urban 5 Janakeeya Hotel 55 0 0 15 Alappuzha Chennam Pallippuram Friends Chennam pallipuram panchayath -

“Assessment of Plant Diversity Including Aquatic Flora, Riparian Vegetation Etc
Impact of Floods/ Landslides on Biodiversity “Assessment of Plant diversity including Aquatic flora, Riparian vegetation etc. in the flood/ Landslides affected areas of Chaliyar, Korapuzha and Kuttiyadi rivers” Final Report Submitted to Kerala State Biodiversity Board KSCSTE - MALABAR BOTANICAL GARDEN AND INSTITUTE FOR PLANT SCIENCES 1 2 Acknowledgement The success and final outcome of this project required lot of guidance and assistance from many people and I am extremely privileged to have got this all along the completion of this project. I thank the Director for providing all support and guidance to complete the project. I heartily thank Dr. Anoop P. Balan and Dr. Anoop K.P. of KSCSTE- MBGIPS who assisted us in completing this project. I would not forget to remember the encouragement and timely support and guidance of Prof. P. V. Madhusoodanan and Dr. P.N. Krishnan (Emeritus Scientists, MBGIPS) in this regard. I owe my deep gratitude to the authority and staff of Local Self Governing Departments of Kozhikode district who guided us all along, till the completion of our project by providing all the necessary information. I would also like to extend our sincere esteems to all staff of the institution for their timely support. Special thanks to Dr. Deepu Sivadas, KSCSTE-JNTBGRI for assisting us in GIS mapping of the study area. The support from Kerala State Biodiversity board and Kerala State Council for Science, Technology and Environment authorities are also duly acknowledged. Dr. N. S. Pradeep 3 4 Contents Sl. No Title 1 Introduction 1.1. Importance of Riparian ecosystem and vegetation 1.2.