Summer 1983 Gems & Gemology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CENTRAL UNIVERSITY of KASHMIR List of Eligible Candidates
CENTRAL UNIVERSITY OF KASHMIR Employment Notification No. 01 of 2018 Dated: 07-02-2018 & 12 of 2016 Dated: 28-12-2016 List of Eligible Candidates Post: MTS/Peon/Office Attendant (02-UR) Post Code: WT-13-2019-CUK Centre Code: G01 & G02 Date of Written Test: 30.06.2019 Session-1: Paper-A (Objective) 02:30pm to 04:00pm Session-2: Paper-B (Descriptive) 04:30pm to 06:00pm Venue: a) Candidates whose Roll No.s from 19131600 to 19132179 shall be at Central University of Kashmir, Green Campus, Opposite Degree College, Ganderbal b) Candidates whose Roll No.s from 19132180-19132274 shall be at Central University of Kashmir, Science & Arts Campus, Old Hospital, Ganderbal- 191201 (J&K) Note: Using below-mentioned Hall Ticket Number, the candidates are informed to download Hall Ticket from the University website w.e.f 28-06-2019 Hall Ticket No. Name of the Applicant Category 19131600 Ms Humira Akhter D/o Mohammad Yousuf Khan UR R/o Iqbal Colony Dialgam Anantnag-192210 19131601 Ms Shahnaza Yousuf D/o Mohammad Yousuf Khan UR R/o Iqbal Colony Dialgam Anantnag-192210 19131602 MsCell:871599247/9596439917 Bismah Rashid D/o Abdul Rashid UR R/o Shaheed Gunj Near Custodian Office Srinagar-190001 19131603 MsCell:9797930029/9622511370 Tabasum Rashid D/o Abdul Rashid Khan UR R/o Shaheed Gunj Near Custodian Office Srinagar-190001 19131604 MrCell:9622829426 Zahoor Ahmad Shan S/o Ali Mohd Shan UR R/o Zungalpora Pahloo Kulgam-192231 19131605 MrCell:9622726400/9797037173 Idrees Hussain Khan S/o Nasir Hussain Khan UR R/o Hassan Abad Rainawari Srinagar-190003 19131606 -

Page11 Local.Qxd (Page 1)
TUESDAY, JUNE 9, 2020 (PAGE 8) DAILY EXCELSIOR, JAMMU Former MLC takes stock Wind storm hits Kishtwar, of people’s problems Doda; power snapped Excelsior Correspondent PHE, PDD, RDD and PWD to Excelsior Correspondent water entered many houses and prepare a detailed project report shops as well causing damage. JAMMU, June 8: Former for various works, highlighted JAMMU, June 8: The tin A senior official said that MLC Vikram Randhawa today by the locals. He assured the roofs of many houses were dam- similar reports have been received visited Vishal Colony of people that he would personally aged and trees uprooted in from Bhaderwah town and other Babliana area in Gandhi Nagar take up the matter with the Kishtwar and Bhaderwah towns parts of district Doda. He said due constituency and took stock of respective departments for early besides other areas of Doda and to rain the Doda-Kishtwar road the problems being faced by the redressal, so that, the people may VDC members during a protest at Doda on Monday. Kishtwar districts late this near Drabshalla sliding zone has inhabitants. get a sigh of relief. —Excelsior/Rafi Choudhary During the visit, the locals Randhawa said that the pace informed him that they were fac- of development has been affect- ing hardships due to absence of ed due to COVID-19 pandemic VDC members demand release of salaries CUK VC chairing meeting of Planning & Monitoring Board basic amenities such as irregular but now slowly and steadily the Excelsior Correspondent to which our families are suffer- on Monday. DODA, June 8: Members of ing," they claimed. -

Kala Zeera (Bunium Persicum Bioss.): a Kashmirian High Value Crop
Turk J Biol 33 (2009) 249-258 © TÜBİTAK doi:10.3906/biy-0803-18 Kala zeera (Bunium persicum Bioss.): a Kashmirian high value crop Parvaze A. SOFI1, Nazeer. A. ZEERAK2, Parmeet SINGH2 1Directorate of Research, SKUAST-K, Srinagar, J&K, 191121, INDIA 2Division of Plant Breeding & Genetics, SKUAST-K, Srinagar, J&K, 191121, INDIA Received: 31.03.2009 Abstract: Kala zeera is a high value, low volume, and under-exploited spice crop that grows in mountainous regions of Kashmir in the Himalayas. It has received very little attention in terms of development, standardization of production technology, and plant protection management practices. Sher-e Kashmir University of Agriculture Sciences and Technology (SKUAST-K) and other organizations have instituted programs for systematic improvement of Kala zeera. In this paper, we offer a synopsis of the latest work being done in promoting this high value crop, which would have a beneficial effect for the encouragement of economic activity in the Himalayas. Key words: Bunium persicun, Apiaceae, spice Kala zeera (Bunium persicum Bioss.): Kaşmir Himalaya bölgesi için pahalı bir baharat Özet: Kala zeera Kaşmir Himalayalarında çok az sayıda bulunan fazla incelenmemiş bir baharat bitksidir. Varyete geliştirme, üretim teknolojilerinin satandardizasyonu ve bitki koruma uygulamaları açısından pek ilgilenilmemiş bir bitkidir. Kala zeera baharatının sistematik olarak geliştirilmesi için SKUAST-K ve else where, programı kullanılmıştır. Bu çalışmada bizim üniversite ve diğer yerlerde Himalaya dağlarında yaşayan insanlara ekonomik olarak büyük fayda sağlayacak baharatın değerini artırmak için yapılan çalışmalar özetlenmiştir. Anahtar sözcükler: Bunium persicun, Apiaceae, baharat Introduction mostly aromatic herbs dispersed throughout the Kala zeera (Bunium persicum Bioss.) is a high value world especially in northern hemisphere (1). -
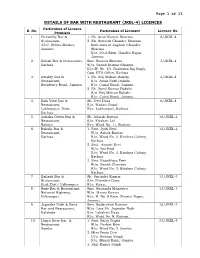
JKEL-4) LICENCES Particulars of Licence S
Page 1 of 13 DETAILS OF BAR WITH RESTAURANT (JKEL-4) LICENCES Particulars of Licence S. No. Particulars of Licensee Licence No. Premises 1. Piccadilly Bar & 1. Sh. Arun Kumar Sharma 2/JKEL-4 Restaurant, 2. Sh. Romesh Chander Sharma 23-C, Nehru Market, both sons of Jagdish Chander Jammu Sharma R/o. 20-A Extn. Gandhi Nagar, Jammu. 2. Satish Bar & Restaurant, Smt. Suman Sharma 3/JKEL-4 Kathua W/o. Satish Kumar Sharma R/o W. No. 10, Chabutra Raj Bagh, Opp. ETO Office, Kathua 3. Kwality Bar & 1. Sh. Brij Mohan Bakshi 4/JKEL-4 Restaurant, S/o. Amar Nath Bakshi Residency Road, Jammu R/o. Canal Road, Jammu. 2. Sh. Sunil Kumar Bakshi S/o. Brij Mohan Bakshi R/o. Canal Road, Jammu. 4. Ravi View Bar & Sh. Devi Dass 8/JKEL-4 Restaurant, S/o. Madan Gopal Lakhanpur, Distt. R/o. Lakhanpur, Kathua. Kathua. 5. Ashoka Green Bar & Sh. Adarsh Rattan 10/JKEL-4 Restaurant, S/o. Krishan Lal Rajouri R/o. Ward No. 11, Rajouri. 6. Bakshi Bar & 1. Smt. Jyoti Devi 12/JKEL-4 Restaurant, W/o. Ashok Kumar Kathua. R/o. Ward No. 3, Krishna Colony, Kathua. 2. Smt . Amarti Devi W/o. Sat Paul R/o. Ward No. 3, Krishna Colony, Kathua. 3. Smt. Kaushlaya Devi W/o. Satish Chander R/o. Ward No. 3, Krishna Colony, Kathua. 7. Kailash Bar & Sh. Surinder Kumar 13/JKEL-4 Restaurant, S/o. Pitamber Dass Kud, Distt. Udhampur. R/o. Katra. 8. Roxy Bar & Restaurant, Smt. Sunanda Mangotra 14/JKEL-4 National Highway, W/o. -

Paddar ) Technical Education Department Our Motto “ Skilled Hands Secured Future ” Website : Email : [email protected]
Government of Jammu & Kashmir Industrial Training Institute ( Paddar ) Technical Education Department our motto “ skilled hands secured future ” website : www.itipaddar.com email : [email protected] Correspondence Address : Ghulab Garh, Paddar, Kishtwar, Jammu, J&K, India. Pin code : 180001 Extension in Admission 2018 – 19 ( Govt. ITI Paddar ) Applications (admission forms) are invited from the Permanent Residents of Jammu and Kashmir for undergoing training in this Institute ( ITI Paddar ) in below detailed trades, for the Session 2018 – 19 The admission forms can be had from office of Govt. ITI Kishtwar or Govt. ITI Paddar the upto 4th July 2018, upto 4.00pm against cash payment of Rs. 40/= or can be downloaded from Institute website ( www.itikishtwar.com &/or www.itipaddar.com ), in latter case cost of Form Rs. 40/- shall be paid at the time of submission of Admission form. The last date for receipt of application forms duly complete in all respects is 7th July 2018, 3.30pm. No forms will be entertained after due date and in absence of self-attested Photocopy of below mentioned documents. a) State Subject b) Date of Birth Certificate. c) Marks Certificate, of entry level qualification, only d) Category Certificate ( SC/ST/Ex. Serviceman/Physically Handicap ) if any e) Two Self Attested Photographs The selection list of the candidates as per the merit will be made available on our official website www.itikishtwar.com &/or www.itipaddar.com and on office notice board. Tentative date for publication of Selection List is 4th Week of July 2018, subject to further extension in admission. Tentative date for starting the academic session/classwork will be 1 week of August 2018. -

Pradhan Mantri Gram Sadak Yojana (PMGSY)
Scheme wise Expenditure under Programme Fund for the Financial Year 2020-2021 (Rs.in Lakhs) Total Expenditure w.e.f 1-4- S NO. Name of PIU Name of Scheme Package No. 2020 to till date 1 Shiva to Yemran JK03-184 0.99 2 Dudbugh to Takiya Yousf Shah JK03-186 66.09 3 L026-Authoora to Thindam Bala JKO3-226 44.18 4 Muqaam Shaheed Mir to Cherhar JK03-165 131.56 5 L036-Wagoora to Durhama JK03-239 16.93 6 L033-Kilwara to Gooricheck JK03-225 83.98 7 LO28- Hatchapora to Chatoosa JK03-220 216.40 8 L044-Srinager to Baramulla uri to Namblan JK03-218 327.46 9 LO25-Trikolbal to Gund Ibrahim JK03-234 123.67 10 L056-Pachar to Masjid Angan JK03-263 75.46 11 LO4-T04 Km 14th to Punipora JK03-222 81.84 12 Tangmarg to Mayan JK03-202 47.15 13 L024-Dildarpora to Wanikhal JK03-243 121.76 14 L035-Fruit Mundi to Block boundary PMGSY II JK03-2012 466.61 15 L031-Km 2nd to Block Boundary JK03-221 249.71 16 L024-Srinager Baramulla Uri km 46th to Singhpora JK03-219 145.98 17 Muqaam Shaheed Mir to Cherhar Stage II JK03-258 245.24 Baramulla 18 L049-Shiva to Kharma JK03-240 121.59 19 Chatoosa to Tangmulla JK03-212 23.60 20 Kakawthal to Branner JK03-189 42.48 21 Delina to Check Delina JK03-167 1.54 22 Kakawthal to Branner stage II JK03-262 98.59 23 Shiva to Yemran Stage II JK03-257 15.65 24 Drang to Gogaldara JK03-54 91.86 25 Km 5th of L021 to Kutipora JK03-203 68.24 26 L046-Kohilna to Rangwar Stage II JK03-261 72.13 27 L037 Kawcheck to waripora JK03-224 108.15 28 L041-Zaloora to Gujarpati JK03-244 89.85 29 Km 7th to Behrampora JK03-97 0.97 30 Lisan to Satrina PMGSY II JK03-2008 -

Sr. Form No. Name Parentage Address District Category MM MO
Modified General Merit list of candidates who have applied for admission to B.Ed. prgoramme (Kashmir Chapter) offered through Directorate of Distance Education, University of Kashmir session-2018 Sr. Form No. Name Parentage Address District Category MM MO %age 1 1892469 TABASUM GANI ABDUL GANI GANAIE NAZNEENPORA TRAL PULWAMA OM 1170 1009 86.24 2 1898382 ZARKA AMIN M A PAMPORI BAGH-I-MEHTAB SRINAGAR OM 10 8.54 85.40 3 1891053 MAIDA MANZOOR MANZOOR AHMAD DAR BATENGOO KHANABAL ANANTNAG ANANTNAG OM 500 426 85.20 4 1892123 FARHEENA IFTIKHAR IFTIKHAR AHMAD WANI AKINGAM ANANTNAG ANANTNAG OM 1000 852 85.20 5 1891969 PAKEEZA RASHID ABDUL RASHID WANI SOGAM LOLAB KUPWARA OM 10 8.51 85.10 6 1893162 SADAF FAYAZ FAYAZ AHMAD SOFAL SHIRPORA ANANTNAG OM 100 85 85.00 BASRAH COLONY ELLAHIBAGH 7 1895017 ROSHIBA RASHID ABDUL RASHID NAQASH BUCHPORA SRINAGAR OM 10 8.47 84.70 8 1894448 RUQAYA ISMAIL MOHAMMAD ISMAIL BHAT GANGI PORA, B.K PORA, BADGAM BUDGAM OM 10 8.44 84.40 9 1893384 SHAFIA SHOWKET SHOWKET AHMAD SHAH BATAMALOO SRINAGAR OM 10 8.42 84.20 BABA NUNIE GANIE, 10 1893866 SAHREEN NIYAZ MUNSHI NIYAZ AHMAD KALASHPORA,SRINAGAR SRINAGAR OM 900 756 84.00 11 1893858 UZMA ALTAF MOHD ALTAF MISGAR GULSHANABAD K.P ROAD ANANTNAG ANANTNAG OM 1000 837 83.70 12 1893540 ASMA RAMZAN BHAT MOHMAD RAMZAN BHAT NAGBAL GANDERBAL GANDERBAL OM 3150 2630 83.49 13 1895633 SEERATH MUSHTAQ MUSHTAQ AHMED WANI DEEWAN COLONY ISHBER NISHAT SRINAGAR OM 1900 1586 83.47 14 1891869 SANYAM VIPIN SETHI ST.1 FRIENDS ENCLAVE FAZILKA OTHER STATE OSJ 2000 1666 83.30 15 1895096 NADIYA AHAD ABDUL AHAD LONE SOGAM LOLAB KUPWARA OM 10 8.33 83.30 16 1892438 TABASUM ASHRAF MOHD. -

Notification No. 51 – PSC (DR-S) of 2018 Dated: 30.01.2018
Page 1 of 69 Subject: Select List for the posts of Medical Officer (Allopathic) in Health & Medical Education Department. Notification No. 51 – PSC (DR-S) of 2018 Dated: 30.01.2018 Whereas, the Health & Medical Education Department referred 371 posts (OM:213, RBA:74, SC:29, ST:37, ALC:11 & SLC:07) of Medical Officer to the Public Service Commission for being filled up from amongst the suitable candidates; and Whereas, the Commission notified these posts vide Notification No. 01-PSC (DR-P) of 2017 dated 27.03.2017; and Whereas, in response to the above notification, 2883 applications were received; and Whereas, the written test of the candidates for selection was conducted on 26.06.2016 in which 2452 candidates appeared. The result of the written test was declared vide Notification No. PSC/Exam/2017/79 dated: 14.12.2017 in pursuance of Rule 32(a) of the J&K Public Service Commission (Conduct of Examinations) Rules, 2005 and Rule 40 of the J&K Public Service Commission (Business & Procedure) Rules, 1980 as amended from time to time and 1158 candidates were declared to have qualified the written test and called for interview; and Whereas, 01 more candidate was allowed to participate in the interview on the directions of the Hon’ble High Court in SWP No. 2834/2017, MP No.01/2017 titled Nidhi Priya Vs State of J&K & Ors. vide its order dated:30.12.2017.Her result has not been declared as per Court Orders. Whereas, the interviews of the shortlisted candidates were conducted w.e.f. -

Industrial Training Institute ( Paddar )
Government of Jammu & Kashmir Industrial Training Institute ( Paddar ) Technical Education Department our motto “ skilled hands secured future ” website : www.itipaddar.com email : [email protected] Correspondence Address : Ghulab Garh, Paddar, Kishtwar, Jammu, J&K, India. Pin code : 182204 Order No :- 17 of 2018 Date :- 12 – 07 - 2018 Subject : Committee for Admission Process. The below official will be the admission committee of this Institute. This committee shall become operative from the date of issuance of this order. S. No Name of Official Designation 1 Mr. Akhter Hussain ( Supervisor ) Chairman 2 Mr. Jugal Kishore Member 3 Mr. Muhammad Amin Member 4 Mr. Sandeep Sen Member Secretary 5 Any other Member Nominated by HOI. - Committee will take-over the charge of already existing admission activity of this Institute, such as sale and receipt of forms and compilation of list etc. The main line of activity of this committee will be :- 1) Framing of General List, strictly as per merit and rules. 2) Framing & Publication of Selection List as per rules. 3) Counseling of Trainees who get admission in this Institute. 4) Counseling of Trainees who have applied for highly responsive trades and donot fall in the merit. 5) To ensure the amount received from sale of forms, admission fee, Caution money, Students welfare, Library, etc are accounted for to the respective bank accounts on daily basis. 6) Any other directions issued time to time. No. : ITI / Pdr / Ktwr - C / 18 / Trg / 506 - 15 Date : 12 / 07 / 2018 Sd/- Imran Wajahat Principal Govt. Industrial Training Institute, Kishtwar ( Additional Charge of Govt. ITI Paddar ) Technical Education Department Copy for information to : 1) Director, Technical Education Department, Srinagar, Govt. -

MIT Japan Program Working Paper 01.10 the GLOBAL COMMERCIAL
MIT Japan Program Working Paper 01.10 THE GLOBAL COMMERCIAL SPACE LAUNCH INDUSTRY: JAPAN IN COMPARATIVE PERSPECTIVE Saadia M. Pekkanen Assistant Professor Department of Political Science Middlebury College Middlebury, VT 05753 [email protected] I am grateful to Marco Caceres, Senior Analyst and Director of Space Studies, Teal Group Corporation; Mark Coleman, Chemical Propulsion Information Agency (CPIA), Johns Hopkins University; and Takashi Ishii, General Manager, Space Division, The Society of Japanese Aerospace Companies (SJAC), Tokyo, for providing basic information concerning launch vehicles. I also thank Richard Samuels and Robert Pekkanen for their encouragement and comments. Finally, I thank Kartik Raj for his excellent research assistance. Financial suppport for the Japan portion of this project was provided graciously through a Postdoctoral Fellowship at the Harvard Academy of International and Area Studies. MIT Japan Program Working Paper Series 01.10 Center for International Studies Massachusetts Institute of Technology Room E38-7th Floor Cambridge, MA 02139 Phone: 617-252-1483 Fax: 617-258-7432 Date of Publication: July 16, 2001 © MIT Japan Program Introduction Japan has been seriously attempting to break into the commercial space launch vehicles industry since at least the mid 1970s. Yet very little is known about this story, and about the politics and perceptions that are continuing to drive Japanese efforts despite many outright failures in the indigenization of the industry. This story, therefore, is important not just because of the widespread economic and technological merits of the space launch vehicles sector which are considerable. It is also important because it speaks directly to the ongoing debates about the Japanese developmental state and, contrary to the new wisdom in light of Japan's recession, the continuation of its high technology policy as a whole. -

05/31/2017 Taylor County Acctpa21 Time: 16:10:10 Check Register − Disbursement Fund
PAGE NUMBER: 1 DATE: 05/31/2017 TAYLOR COUNTY ACCTPA21 TIME: 16:10:10 CHECK REGISTER − DISBURSEMENT FUND SELECTION CRITERIA: transact.ck_date between ’20170501 00:00:00.000’ and ’20170531 00:00:00.000’ ACCOUNTING PERIOD: 8/17 FUND − 411 − GENERAL CLEARING CASH ACCT CHECK NO ISSUE DT VENDOR NAME DEPARTMENT −−−−−DESCRIPTION−−−−−− SALES TAX AMOUNT 1001 1015498 05/02/17 1671 A−1 VACUUMS 6570 WINSOR REPAIR 0.00 45.00 1001 1015499 05/02/17 4178 BRANDON ABBOTT 6010 DAYTON 0.00 76.00 1001 1015500 05/02/17 2088 ABERCROMBIE PEST CONTROL 3075 PEST CNTRL SRV 0.00 45.00 1001 1015501 05/02/17 1063 ABILENE AUTO GLASS 6010 14−17 TAHOE 0.00 389.00 1001 1015502 05/02/17 1709 ABILENE DIAGNOSTIC, PLLC 7010 VARIOUS PEOPLE 0.00 177.89 1001 1015503 05/02/17 2170 ABILENE GENERAL TIRE CO. 5400 FLT RPR,TIRE SEALNT 0.00 47.50 1001 1015504 05/02/17 2021 ABILENE HYDRAULICS, LLC 5300 HYD CYLNDR RPR 0.00 180.00 1001 1015505 05/02/17 1087 BMC ABILENE LUMBER 5015 2X6 0.00 131.66 1001 1015506 05/02/17 1089 ABILENE MAINTENANCE SUPP 6570 FILTERS 0.00 94.00 1001 1015506 05/02/17 1089 ABILENE MAINTENANCE SUPP 6570 TISSUE,TWL,LNR,GLOVES 0.00 241.60 TOTAL CHECK 0.00 335.60 1001 1015507 05/02/17 1097 APSCO 6550 VALVE KIT,PVC,HOSE 0.00 70.78 1001 1015507 05/02/17 1097 APSCO 6550 TEE,BUSHING,GLUE 0.00 290.63 1001 1015507 05/02/17 1097 APSCO 5030 TEE,FAUCET,COUP 0.00 141.43 1001 1015507 05/02/17 1097 APSCO 5030 ANGLE STOP, NIPPLE 0.00 28.86 1001 1015507 05/02/17 1097 APSCO 6550 BUSHING 0.00 85.37 1001 1015507 05/02/17 1097 APSCO 6550 UNION,MIP,TUBING 0.00 117.54 1001 1015507 05/02/17 -

<> CRONOLOGIA DE LOS SATÉLITES ARTIFICIALES DE LA
1 SATELITES ARTIFICIALES. Capítulo 5º Subcap. 10 <> CRONOLOGIA DE LOS SATÉLITES ARTIFICIALES DE LA TIERRA. Esta es una relación cronológica de todos los lanzamientos de satélites artificiales de nuestro planeta, con independencia de su éxito o fracaso, tanto en el disparo como en órbita. Significa pues que muchos de ellos no han alcanzado el espacio y fueron destruidos. Se señala en primer lugar (a la izquierda) su nombre, seguido de la fecha del lanzamiento, el país al que pertenece el satélite (que puede ser otro distinto al que lo lanza) y el tipo de satélite; este último aspecto podría no corresponderse en exactitud dado que algunos son de finalidad múltiple. En los lanzamientos múltiples, cada satélite figura separado (salvo en los casos de fracaso, en que no llegan a separarse) pero naturalmente en la misma fecha y juntos. NO ESTÁN incluidos los llevados en vuelos tripulados, si bien se citan en el programa de satélites correspondiente y en el capítulo de “Cronología general de lanzamientos”. .SATÉLITE Fecha País Tipo SPUTNIK F1 15.05.1957 URSS Experimental o tecnológico SPUTNIK F2 21.08.1957 URSS Experimental o tecnológico SPUTNIK 01 04.10.1957 URSS Experimental o tecnológico SPUTNIK 02 03.11.1957 URSS Científico VANGUARD-1A 06.12.1957 USA Experimental o tecnológico EXPLORER 01 31.01.1958 USA Científico VANGUARD-1B 05.02.1958 USA Experimental o tecnológico EXPLORER 02 05.03.1958 USA Científico VANGUARD-1 17.03.1958 USA Experimental o tecnológico EXPLORER 03 26.03.1958 USA Científico SPUTNIK D1 27.04.1958 URSS Geodésico VANGUARD-2A