Regulation of Diapause Entry and Termination in the Swede Midge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predatory Gall Midge (Unofficial Common Name), Feltiella Acarisuga (Vallot) (Insecta: Diptera: Cecidomyiidae)1 Ryan S
EENY269 Predatory Gall Midge (Unofficial Common Name), Feltiella acarisuga (Vallot) (Insecta: Diptera: Cecidomyiidae)1 Ryan S. Osborne, Norman C. Leppla, and Lance S. Osborne2 Introduction Feltiella ithacae Feltiella quadrata (Gagné 1995) The predatory gall midge, Feltiella acarisuga (Vallot), is one of the most effective and widespread natural enemies of spider mites (Tetranychidae) (Gagné 1995). Because of their Distribution flying and prey-detecting capabilities, and high feeding The genus Feltiella is virtually cosmopolitan and contains potential, it is considered an important natural enemy of 10 species: Feltiella acarisuga (worldwide, except for the the twospotted spider mite, Tetranychus urticae Koch, in a Neotropical Region), Feltiella pini (Felt) (North and Central number of cropping systems (Opit et al. 1997; Refaei and America, West Indies, Australia), Feltiella curtistylus Mohamed 2013). It is also known to feed on other pest Gagné (Brazil), Feltiella occidentalis (Felt) (US—California; mites, including brown almond mite, Bryobia rubrioculus Japan—Honshu), Feltiella acarivora (Zehnter) (Indonesia- Scheuten; carmine spider mite, Tetranychus cinnabarinus Java; Australia), Feltiella insularis (Felt) (US—Illinois, New Boisduval; and European red mite, Panonychus ulmi Koch. Jersey, Florida; West Indies, Colombia, Argentina), Feltiella Feltiella acarisuga could be particularly useful for integrated reducta Felt (US—New York), Feltiella ligulata Gagné (Cape pest management of spider mites that attack greenhouse Verde Island), Feltiella kanchanjungaensis (India—West crops (Gillespie et al. 1998). Bengal) and Feltiella tetranychi (Germany) (Gagné 1995, 2010). Feltiella acarisuga is the most widely distributed Synonymy species in the genus and is listed from the US, Canada, Finland, Germany, United Kingdom, Switzerland, Italy, Cecidomyia acarisuga Morocco, Greece, Israel, India, Sri Lanka, Taiwan, Japan, Mycodiplosis minuta and New Zealand. -

Dipterists Forum
BULLETIN OF THE Dipterists Forum Bulletin No. 76 Autumn 2013 Affiliated to the British Entomological and Natural History Society Bulletin No. 76 Autumn 2013 ISSN 1358-5029 Editorial panel Bulletin Editor Darwyn Sumner Assistant Editor Judy Webb Dipterists Forum Officers Chairman Martin Drake Vice Chairman Stuart Ball Secretary John Kramer Meetings Treasurer Howard Bentley Please use the Booking Form included in this Bulletin or downloaded from our Membership Sec. John Showers website Field Meetings Sec. Roger Morris Field Meetings Indoor Meetings Sec. Duncan Sivell Roger Morris 7 Vine Street, Stamford, Lincolnshire PE9 1QE Publicity Officer Erica McAlister [email protected] Conservation Officer Rob Wolton Workshops & Indoor Meetings Organiser Duncan Sivell Ordinary Members Natural History Museum, Cromwell Road, London, SW7 5BD [email protected] Chris Spilling, Malcolm Smart, Mick Parker Nathan Medd, John Ismay, vacancy Bulletin contributions Unelected Members Please refer to guide notes in this Bulletin for details of how to contribute and send your material to both of the following: Dipterists Digest Editor Peter Chandler Dipterists Bulletin Editor Darwyn Sumner Secretary 122, Link Road, Anstey, Charnwood, Leicestershire LE7 7BX. John Kramer Tel. 0116 212 5075 31 Ash Tree Road, Oadby, Leicester, Leicestershire, LE2 5TE. [email protected] [email protected] Assistant Editor Treasurer Judy Webb Howard Bentley 2 Dorchester Court, Blenheim Road, Kidlington, Oxon. OX5 2JT. 37, Biddenden Close, Bearsted, Maidstone, Kent. ME15 8JP Tel. 01865 377487 Tel. 01622 739452 [email protected] [email protected] Conservation Dipterists Digest contributions Robert Wolton Locks Park Farm, Hatherleigh, Oakhampton, Devon EX20 3LZ Dipterists Digest Editor Tel. -

Arthropod Pest Management in Greenhouses and Interiorscapes E
Arthropod Pest Management in Greenhouses and Interiorscapes E-1011E-1011 OklahomaOklahoma CooperativeCooperative ExtensionExtension ServiceService DivisionDivision ofof AgriculturalAgricultural SciencesSciences andand NaturalNatural ResourcesResources OklahomaOklahoma StateState UniversityUniversity Arthropod Pest Management in Greenhouses and Interiorscapes E-1011 Eric J. Rebek Extension Entomologist/ Ornamentals and Turfgrass Specialist Michael A. Schnelle Extension Ornamentals/ Floriculture Specialist ArthropodArthropod PestPest ManagementManagement inin GreenhousesGreenhouses andand InteriorscapesInteriorscapes Insects and their relatives cause major plant ing a hand lens. damage in commercial greenhouses and interi- Aphids feed on buds, leaves, stems, and roots orscapes. Identification of key pests and an un- by inserting their long, straw-like, piercing-suck- derstanding of appropriate control measures are ing mouthparts (stylets) and withdrawing plant essential to guard against costly crop losses. With sap. Expanding leaves from damaged buds may be tightening regulations on conventional insecti- curled or twisted and attacked leaves often display cides and increasing consumer sensitivity to their chlorotic (yellow-white) speckles where cell con- use in public spaces, growers must seek effective tents have been removed. A secondary problem pest management alternatives to conventional arises from sugary honeydew excreted by aphids. chemical control. Management strategies cen- Leaves may appear shiny and become sticky from tered around -

1 an Example of Parasitoid Foraging: Torymus Capite
1 AN EXAMPLE OF PARASITOID FORAGING: TORYMUS CAPITE (HUBER; HYMEMOPTERA: TORYMIDAE [CHALCIDOIDEA]) ATTACKING THE GOLDENROD GALL-MIDGE ASTEROMYIA CARBONIFERA (O. S.; DIPTERA: CECIDOMYIIDAE) Richard F. Green Department of Mathematics and Statistics University of Minnesota Duluth Duluth, MN 55812 U. S. A. INTRODUCTION Van Alphen and Vet (1986) refer to the work of Arthur E. Weis (1983) on a torymid wasp that attacks a gall midge on goldenrod. This system seems to be quite well-studied, particularly, but not exclusively, by Weis. Van Alphen and Vet point out that the parasitoids tend to attack about the same proportion of hosts in patches (galls) with varying numbers of hosts. This has implications for the foraging strategy that the parasitoids use. In this note I want to do three things: (1) outline the basic biology of the organisms involved, (2) describe the results of a foraging experiment conducted by Weis (1983), and (3) interpret the results in terms of Oaten’s stochastic model of optimal foraging. Arthur E. Weis is coauthor of a book (Abrahamson and Weis 1997) on the biology of a three trophic-level system involving a goldenrod stem-gall maker Eurosta solidaginis, its host plant and its enemies. The work described here is earlier work, done an a different species. The biology of the system The species of greatest interest are the torymid parasitoid Torymus capite, which is a larval parasitoid of the gall midge Asteromyia carbonifera, which itself makes blister-like galls on the leaves of goldenrod, especially Canadian goldenrod, Solidgo canadiensis L. (Compositae). There are three generations of gall midge (and its parasitoids) each year. -

Identified Difficulties and Conditions for Field Success of Biocontrol
Identified difficulties and conditions for field success of biocontrol. 4. Socio-economic aspects: market analysis and outlook Bernard Blum, Philippe C. Nicot, Jürgen Köhl, Michelina Ruocco To cite this version: Bernard Blum, Philippe C. Nicot, Jürgen Köhl, Michelina Ruocco. Identified difficulties and conditions for field success of biocontrol. 4. Socio-economic aspects: market analysis and outlook. Classical and augmentative biological control against diseases and pests: critical status analysis and review of factors influencing their success, IOBC - International Organisation for Biological and Integrated Controlof Noxious Animals and Plants, 2011, 978-92-9067-243-2. hal-02809583 HAL Id: hal-02809583 https://hal.inrae.fr/hal-02809583 Submitted on 6 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. WPRS International Organisation for Biological and Integrated Control of Noxious IOBC Animals and Plants: West Palaearctic Regional Section SROP Organisation Internationale de Lutte Biologique et Integrée contre les Animaux et les OILB Plantes Nuisibles: -

DIPTERA: CECIDOMYIIDAE) in RESPONSE to Llmlted AVAILABILITY of ITS PREY Tetranychus Urticae KOCH (ACARI: TETRANYCHIDAE)
LARVAL DEVELOPMENT OF THE PREDATORY MIDGE Feitieila acarisuga VALLOT (DIPTERA: CECIDOMYIIDAE) IN RESPONSE TO LlMlTED AVAILABILITY OF ITS PREY Tetranychus urticae KOCH (ACARI: TETRANYCHIDAE) by Heidi Nadene Sawyer Mc., University of British Columbia, 1991. THESIS SUBMllTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF PEST MANAGEMENT in the Department of Biological Sciences O H.N. Sawyer 1998 SIMON FRASER UNIVERSITY January 1998 All rights reserved. This work may not be reproduced in whole or in part, by photocopy or other rneans, without permission of the author. Bibliothèque nationale du Canada Acquisitions and Acquisitions et Bibliographie Services services bibliographiques 395 Wellington Çtreet 395. rue Wellington Ottawa ON KIA ON4 OttawaON KlAûN4 CaMdct canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Lhrary of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sen reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/^ de reproduction sur papier ou sur fonnat électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or othenivise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. ABSTRACT In order to evaluate the predatory midge Feltiella acarisuga Vallot as a biological control agent for the two-spotted spider mite Tetranychus uttïcae Koch (TSSM) in greenhouses, information is needed on its performance under conditions of prey limitation. -
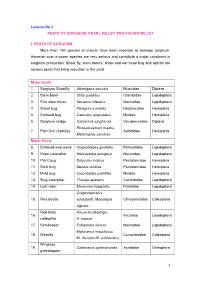
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

Evolutionary Diversification of the Gall Midge Genus Asteromyia
Molecular Phylogenetics and Evolution 54 (2010) 194–210 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evolutionary diversification of the gall midge genus Asteromyia (Cecidomyiidae) in a multitrophic ecological context John O. Stireman III a,*, Hilary Devlin a, Timothy G. Carr b, Patrick Abbot c a Department of Biological Sciences, Wright State University, 3640 Colonel Glenn Hwy., Dayton, OH 45435, USA b Department of Ecology and Evolutionary Biology, Cornell University, E145 Corson Hall, Ithaca, NY 14853, USA c Department of Biological Sciences, Vanderbilt University, Box 351634 Station B, Nashville, TN 37235, USA article info abstract Article history: Gall-forming insects provide ideal systems to analyze the evolution of host–parasite interactions and Received 3 April 2009 understand the ecological interactions that contribute to evolutionary diversification. Flies in the family Revised 17 August 2009 Cecidomyiidae represent the largest radiation of gall-forming insects and are characterized by complex Accepted 9 September 2009 trophic interactions with plants, fungal symbionts, and predators. We analyzed the phylogenetic history Available online 16 September 2009 and evolutionary associations of the North American cecidomyiid genus Asteromyia, which is engaged in a complex and perhaps co-evolving community of interactions with host-plants, fungi, and parasitoids. Keywords: Mitochondrial gene trees generally support current classifications, but reveal extensive cryptic diversity Adaptive diversification within the eight named species. Asteromyia likely radiated after their associated host-plants in the Aste- Fungal mutualism Insect-plant coevolution reae, but species groups exhibit strong associations with specific lineages of Astereae. Evolutionary asso- Cryptic species ciations with fungal mutualists are dynamic, however, and suggest rapid and perhaps coordinated Parasitoid changes across trophic levels. -

Oxidative Stress and Hormesis in Evolutionary Ecology And
David Costantini Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology A Marriage Between Mechanistic and Evolutionary Approaches Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology David Costantini Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology A Marriage Between Mechanistic and Evolutionary Approaches 123 David Costantini Department of Biology University of Antwerp Wilrijk Belgium and Institute for Biodiversity, Animal Health and Comparative Medicine University of Glasgow Glasgow UK ISBN 978-3-642-54662-4 ISBN 978-3-642-54663-1 (eBook) DOI 10.1007/978-3-642-54663-1 Springer Heidelberg New York Dordrecht London Library of Congress Control Number: 2014934114 Ó Springer-Verlag Berlin Heidelberg 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. -

The Searching Behaviour of the Predatory Midge Larva
THE SEARCHING BEHAVIOUR OF THE PREDATORY MIDGE LARVA, Feltiella acacatisuga VALLOT (DIPTERA: CECIDOMYIIDAE), IN RESPONSE TO THE DENSlTY AND DISTRIBUTION OF ITS PREY, T&anychus u&ue KOCH (ACARI: TETRANYCHIDAE) Marc Johnston B .Sc. McGill University, 1993 THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF PEST MANAGEMENT in the Department of Biological Sciences Q Marc Johnston SIMON FRASER UNIVERSITY July 1997 All rights reserved. This work may not be reproduced in whole or in part, by photocopy or other means, without permission of the author. National Library Biblioth&que nationale of Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellmgton Street 395, rue Wellington OnawaON KlAON4 Onawa ON K 1 A OW Canada Canada 4 bur !@ Norre retefence c. The author has granted a non- L'auteur a accorde une licence non exclusive licence allowing the exclusive permettant a la National Library bf Canada to Bibliotheque nationale du Canada de reproduce, loan, distribute or sell reproduire, przter, distri buer ou copies of this thesis in microform, vendre des copies de cette these sous paper or electronic formats. la forrne de microfiche/film, de reproduction sur papier ou sur format electronique. The author retains ownershp of the L'auteur conserve la propriete du copyright in hsthesis. Neither the droit d'auteur qui protege cette these. thesis nor substantial extracts from it Ni lalhkse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent etre imprimes reproduced without the author's ou autrement reprodiits sans son permission. autorisation. -

ECOLOGICAL FACTORS EXPLAINING GENETIC DIFFERENTIATION in APHIDOMORPHA ASSOCIATED with PECAN and WATER HICKORY TREES a Dissertati
ECOLOGICAL FACTORS EXPLAINING GENETIC DIFFERENTIATION IN APHIDOMORPHA ASSOCIATED WITH PECAN AND WATER HICKORY TREES A Dissertation by KYLE EDWARD HARRISON Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, Raul F. Medina Committee Members, Thomas J. DeWitt Cecilia Tamborindeguy Aaron M. Tarone Head of Department, David W. Ragsdale May 2017 Major Subject: Entomology Copyright 2017 Kyle Harrison ABSTRACT Host-associated differentiation (HAD) is a form of ecologically mediated host-race formation between parasite populations. Since HAD can ultimately lead to speciation, it has been proposed as a way to account for the vast species diversity observed in parasitic arthropods. However, the importance of HAD to species diversity is unclear because the factors explaining the occurrence of HAD are only partially understood. Still, there are several examples of parasite-host case study systems for which there is a known cause of reproductive isolation between host-associated parasite populations. Thus, several biological and ecological factors (e.g., immigrant inviability or allochrony) have been proposed as explanatory factors for HAD occurrence. The body of research presented here represents the first quantitative assessment of the generalized relationship between HAD occurrence and the incidence of the proposed explanatory factors. This research was supported by field experiments that assessed the co-occurrence of HAD and particularly important explanatory factors. These experiments were conducted in a community of Aphidomorpha species living on pecan and water hickory trees. I found that HAD can be explained in general based on the incidence of specific explanatory factors (i.e. -

ECOLOGICAL FACTORS AFFECTING the ESTABLISHMENT of the BIOLOGICAL CONTROL AGENT Gargaphia Decoris DRAKE (HEMIPTERA: TINGIDAE)
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. ECOLOGICAL FACTORS AFFECTING THE ESTABLISHMENT OF THE BIOLOGICAL CONTROL AGENT Gargaphia decoris DRAKE (HEMIPTERA: TINGIDAE) A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Plant Science at Massey University, Manawatu, New Zealand Cecilia María Falla 2017 ABSTRACT The Brazilian lace bug (Gargaphia decoris Drake (Hemiptera:Tingidae)) was released in New Zealand in 2010 for the biological control of the invasive weed woolly nightshade (Solanum mauritianum Scopoli (Solanaceae)). Currently there is scarce information about the potential effect of ecological factors on the establishment of this biological control agent. This study investigated: 1) the effect of maternal care and aggregation on nymphal survival and development; 2) the effect of temperature, photoperiod and humidity on G. decoris performance; and 3) the effect of light intensity on S. mauritianum and G. decoris performance. Maternal care and aggregation are characteristic behaviours of G. decoris. These behaviours have an adaptive significance for the offspring and are key determinants for the survival of the species under natural conditions. Maternal care is reported to increase the survival and development of offspring under field conditions, and higher aggregations to increase the survival of the offspring. However, in this study, maternal care negatively affected the survival and development of the offspring, and higher aggregations had no significant impact on offspring survival.