Cannabinoid Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cannabidiol Attenuates Seizures and Social Deficits in a Mouse Model of Dravet Syndrome
Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome Joshua S. Kaplana, Nephi Stellaa,b,1, William A. Catteralla,1,2, and Ruth E. Westenbroeka,1 aDepartment of Pharmacology, University of Washington, Seattle, WA 98195; and bDepartment of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA 98195 Contributed by William A. Catterall, September 7, 2017 (sent for review July 3, 2017; reviewed by Lori L. Isom, Daniele Piomelli, and Peter C. Ruben) Worldwide medicinal use of cannabis is rapidly escalating, despite 17). Previous work showed that DS symptoms result from the loss- limited evidence of its efficacy from preclinical and clinical studies. of-function of Nav1.1 channels, which selectively reduces sodium Here we show that cannabidiol (CBD) effectively reduced seizures current and excitatory drive in many types of GABAergic inter- and autistic-like social deficits in a well-validated mouse genetic neurons (13, 14, 18–20). Accordingly, targeting the Scn1a mutation model of Dravet syndrome (DS), a severe childhood epilepsy disorder to Nav1.1 channels in forebrain GABAergic interneurons re- caused by loss-of-function mutations in the brain voltage-gated capitulated the DS phenotype and established that hypoexcitability sodium channel NaV1.1. The duration and severity of thermally in- of these interneurons is sufficient to cause the epileptic phenotype duced seizures and the frequency of spontaneous seizures were sub- (21) and autistic-like behaviors (16) observed in DS mice. In con- stantially decreased. Treatment with lower doses of CBD also trast, targeting the Scn1a mutation to excitatory neurons ameliorates improved autistic-like social interaction deficits in DS mice. -

Lifestyle Drugs” for Men and Women
Development of “Lifestyle Drugs” for Men and Women Armin Schultz CRS - Clinical Research Services Mannheim GmbH AGAH Annual Meeting 2012, Leipzig, March 01 - 02 Lifestyle drugs Smart drugs, Quality-of-life drugs, Vanity drugs etc. Lifestyle? Lifestyle-Drugs? Active development? Discovery by chance? AGAH Annual Meeting 2012, Leipzig, March 01 - 02 Lifestyle A lifestyle is a characteristic bundle of behaviors that makes sense to both others and oneself in a given time and place, including social relations, consumption, entertainment, and dress. The behaviors and practices within lifestyles are a mixture of habits, conventional ways of doing things, and reasoned actions „Ein Lebensstil ist [...] der regelmäßig wiederkehrende Gesamtzusammenhang der Verhaltensweisen, Interaktionen, Meinungen, Wissensbestände und bewertenden Einstellungen eines Menschen“ (Hradil 2005: 46) Different definitions in social sciences, philosophy, psychology or medicine AGAH Annual Meeting 2012, Leipzig, March 01 - 02 Lifestyle Many “subdivisions” LOHAS: “Lifestyles of Health and Sustainability“ LOVOS: “Lifestyles of Voluntary Simplicity“ SLOHAS: “Slow Lifestyles of Happiness and Sustainability” PARKOS: “Partizipative Konsumenten“ ……. ……. ……. AGAH Annual Meeting 2012, Leipzig, March 01 - 02 Lifestyle drugs Lifestyle drug is an imprecise term commonly applied to medications which treat non-life threatening and non-painful conditions such as baldness, impotence, wrinkles, or acne, without any medical relevance at all or only minor medical relevance relative to others. Desire for increase of personal well-being and quality of life It is sometimes intended as a pejorative, bearing the implication that the scarce medical research resources allocated to develop such drugs were spent frivolously when they could have been better spent researching cures for more serious medical conditions. -

Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer
cancers Review Cannabinoids and Endocannabinoid System Changes in Intestinal Inflammation and Colorectal Cancer Viktoriia Cherkasova, Olga Kovalchuk * and Igor Kovalchuk * Department of Biological Sciences, University of Lethbridge, Lethbridge, AB T1K 7X8, Canada; [email protected] * Correspondence: [email protected] (O.K.); [email protected] (I.K.) Simple Summary: In recent years, multiple preclinical studies have shown that changes in endo- cannabinoid system signaling may have various effects on intestinal inflammation and colorectal cancer. However, not all tumors can respond to cannabinoid therapy in the same manner. Given that colorectal cancer is a heterogeneous disease with different genomic landscapes, experiments with cannabinoids should involve different molecular subtypes, emerging mutations, and various stages of the disease. We hope that this review can help researchers form a comprehensive understanding of cannabinoid interactions in colorectal cancer and intestinal bowel diseases. We believe that selecting a particular experimental model based on the disease’s genetic landscape is a crucial step in the drug discovery, which eventually may tremendously benefit patient’s treatment outcomes and bring us one step closer to individualized medicine. Abstract: Despite the multiple preventive measures and treatment options, colorectal cancer holds a significant place in the world’s disease and mortality rates. The development of novel therapy is in Citation: Cherkasova, V.; Kovalchuk, critical need, and based on recent experimental data, cannabinoids could become excellent candidates. O.; Kovalchuk, I. Cannabinoids and This review covered known experimental studies regarding the effects of cannabinoids on intestinal Endocannabinoid System Changes in inflammation and colorectal cancer. In our opinion, because colorectal cancer is a heterogeneous Intestinal Inflammation and disease with different genomic landscapes, the choice of cannabinoids for tumor prevention and Colorectal Cancer. -

Cannabinoid-Induced Hypotension and Bradycardia in Rats Is 1 Mediated by CB1-Like Cannabinoid Receptors
0022-3565/97/2813-1030$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 281, No. 3 Copyright © 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A. JPET 281:1030–1037, 1997 Cannabinoid-Induced Hypotension and Bradycardia in Rats Is 1 Mediated by CB1-Like Cannabinoid Receptors KRISTY D. LAKE, DAVID R. COMPTON, KAROLY VARGA, BILLY R. MARTIN and GEORGE KUNOS Department of Pharmacology and Toxicology, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia Accepted for publication February 19, 1997 ABSTRACT 9 Previous studies indicate that the CB1 cannabinoid receptor an- potency was (-)-11-OH-D -THC dimethylheptyl $ (-)-3-[2- tagonist, N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophe- hydroxy-4-(1,1-dimethyl-heptyl)phenyl]-4-[3-hydroxy-propyl]cy- nyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A), in- clohexan-1-ol . (-)-3-[2-hydroxy-4-(1,1-dimethyl-heptyl)phenyl]- hibits the anandamide- and D9-tetrahydrocannabinol- (THC) 4-[3-hydroxy-propyl]cyclohexan-1-ol . THC . anandamide $ induced hypotension and bradycardia in anesthetized rats with a (-)-3-[2-hydroxy-4-(1,1-dimethyl-heptyl)phenyl]-4-[3-hydroxy- potency similar to that observed for SR141716A antagonism of propyl]cyclohexan-1-ol, which correlated well with CB1 receptor THC-induced neurobehavioral effects. To further test the role of affinity or analgesic potency (r 5 0.96-0.99). There was no hypo- CB1 receptors in the cardiovascular effects of cannabinoids, we tension or bradycardia after palmitoylethanolamine or (1)-11-OH- examined two additional criteria for receptor-specific interactions: D9-THC dimethylheptyl. -

Cannabinoids in Dermatology: a Scoping Review
Volume 24 Number 6| June 2018| Dermatology Online Journal || Review 24(6): 1 Cannabinoids in dermatology: a scoping review Lauren R M Eagelston1 MD, Nazanin Kuseh Kalani Yazd1 BS, Ravi R Patel2 MD, Hania K Flaten1 BS, Cory A Dunnick3,4 MD, Robert P Dellavalle3,4 MD PhD MSPH Affiliations: 1University of Colorado School of Medicine, Aurora, Colorado, USA, 2Gwinnett Medical Center, Lawrenceville, Georgia, USA, 3University of Colorado School of Medicine, Department of Dermatology, Aurora, Colorado, 4Denver Veterans Affairs Medical Center (VAMC), Department of Dermatology, Denver, Colorado Corresponding Author: Robert P. Dellavalle MD PhD MSPH, Professor of Dermatology, Chief of Dermatology Service, Department of Veteran Affairs Medical Center, 1055 Clermont Rm 6A-105 Mail Stop 16, Denver, CO 80220, Tel: 303.339.8020 x2475, Email: [email protected] Introduction Abstract The cannabinoids are a diverse class of The therapeutic applications of cannabis and pharmacologically active compounds that are cannabinoids are an increasingly conspicuous topic structurally and biologically similar to the primary as de-criminalization and legalization of these products continues to expand. A limited number of psychoactive compound derived from Cannabis cannabinoid compounds have been approved for a sativa -tetrahydrocannabinol (THC), [1-4]. There specific set of conditions. However, the current role are three classes of cannabinoids. Endogenous of cannabinoids for the treatment of dermatologic cannabinoids (endocannabinoids) occur conditions remains to be defined. We conducted a naturally/are constitutively produced in the bodies review of the current literature to determine the of humans and animals [3, 5, 6]. The most well-known applications of cannabinoids for the therapy of members of this class include 2-arachidonoyl- various skin diseases. -

N-Acyl-Dopamines: Novel Synthetic CB1 Cannabinoid-Receptor Ligands
Biochem. J. (2000) 351, 817–824 (Printed in Great Britain) 817 N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo Tiziana BISOGNO*, Dominique MELCK*, Mikhail Yu. BOBROV†, Natalia M. GRETSKAYA†, Vladimir V. BEZUGLOV†, Luciano DE PETROCELLIS‡ and Vincenzo DI MARZO*1 *Istituto per la Chimica di Molecole di Interesse Biologico, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy, †Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, R. A. S., 16/10 Miklukho-Maklaya Str., 117871 Moscow GSP7, Russia, and ‡Istituto di Cibernetica, C.N.R., Via Toiano 6, 80072 Arco Felice, Napoli, Italy We reported previously that synthetic amides of polyunsaturated selectivity for the anandamide transporter over FAAH. AA-DA fatty acids with bioactive amines can result in substances that (0.1–10 µM) did not displace D1 and D2 dopamine-receptor interact with proteins of the endogenous cannabinoid system high-affinity ligands from rat brain membranes, thus suggesting (ECS). Here we synthesized a series of N-acyl-dopamines that this compound has little affinity for these receptors. AA-DA (NADAs) and studied their effects on the anandamide membrane was more potent and efficacious than anandamide as a CB" transporter, the anandamide amidohydrolase (fatty acid amide agonist, as assessed by measuring the stimulatory effect on intra- hydrolase, FAAH) and the two cannabinoid receptor subtypes, cellular Ca#+ mobilization in undifferentiated N18TG2 neuro- CB" and CB#. NADAs competitively inhibited FAAH from blastoma cells. This effect of AA-DA was counteracted by the l µ N18TG2 cells (IC&! 19–100 M), as well as the binding of the CB" antagonist SR141716A. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

Carboxylesterases: Pharmacological Inhibition Regulated Expression and Transcriptional Involvement of Nuclear Receptors and Other Transcription Factors
CARBOXYLESTERASES: PHARMACOLOGICAL INHIBITION REGULATED EXPRESSION AND TRANSCRIPTIONAL INVOLVEMENT OF NUCLEAR RECEPTORS AND OTHER TRANSCRIPTION FACTORS Yuanjun Shen1, Zhanquan Shi2 and Bingfang Yan2,3 Division of Pharmaceutical Sciences James L. Winkle College of Pharmacy University of Cincinnati Cincinnati, OH 45229 USA. Address Correspondence to: Dr. Bingfang Yan Division of Pharmaceutical Sciences James L. Winkle College of Pharmacy University of Cincinnati Cincinnati, OH 45229 Phone: (513) 558-6297 Fax: (513) 558-3233 E-mail: [email protected] Running Title: Regulated expression and inhibited activity of carboxylesterases FOOTNOTES 1 Current address: Pittsburgh Heart, Lung and Blood Vascular Medicine Institute, University of Pittsburgh Department of Medicine, Pittsburgh, PA 15261, USA. 2 Division of Pharmaceutical Sciences, James L. Winkle College of Pharmacy, University of Cincinnati, Cincinnati, OH 45229, USA 3 To whom correspondence should be addressed. 4 This work was supported by National Institutes of Health Grants R01GM61988 and R01EB018748. 5 Abbreviation used: CAR, Constitutive androstane receptor; CES1, carboxylesterase-1; DEC1, Differentiated embryo chondrocyte-1; 5-FU: Fluorouracil; GR, Glucocorticoid receptor; IL-6, Interlekin- 6; LPS, Lipopolysaccharides; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; p53, Tumor protein p53; PXR, Pregnane X receptor Carboxylesterases (CESs, E.C.3.1.1.1) constitute a large class of enzymes that determine the therapeutic efficacy and toxicity of ester/amide drugs. Without exceptions, all mammalian species studied express multiple forms of carboxylesterases. Two human carboxylesterases, CES1 and CES2, are major contributors to hydrolytic biotransformation. Recent studies have identified therapeutic agents that potently inhibit carboxylesterases-based catalysis. Some of them are reversible whereas others irreversible. The adrenergic antagonist carvedilol, for example, reversibly inhibits CES2 but this carboxylesterase is irreversibly inhibited by orlistat, a popular anti-obesity medicine. -
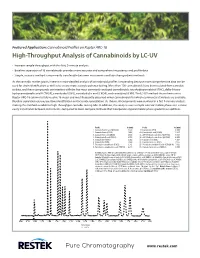
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’S Disease
Molecular Neurobiology https://doi.org/10.1007/s12035-019-1637-8 Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease David Schubert1 & Devin Kepchia1 & Zhibin Liang1 & Richard Dargusch1 & Joshua Goldberg & Pamela Maher1 Received: 30 January 2019 /Accepted: 6 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Finding a therapy for Alzheimer’s disease (AD) is perhaps the greatest challenge for modern medicine. The chemical scaffolds of many drugs in the clinic today are based upon natural products from plants, yet Cannabis has not been extensively examined as a source of potential AD drug candidates. Here, we determine if a number of non-psychoactive cannabinoids are neuroprotective in a novel pre-clinical AD and neurodegeneration drug-screening platform that is based upon toxicities associated with the aging brain. This drug discovery paradigm has yielded several compounds in or approaching clinical trials for AD. Eleven cannabinoids were assayed for neuroprotection in assays that recapitulate proteotoxicity, loss of trophic support, oxidative stress, energy loss, and inflammation. These compounds were also assayed for their ability to remove intraneuronal amyloid and subjected to a structure-activity relationship analysis. Pairwise combinations were assayed for their ability to synergize to produce neuropro- tective effects that were greater than additive. Nine of the 11 cannabinoids have the ability to protect cells in four distinct phenotypic neurodegeneration screening assays, including those using neurons that lack CB1 and CB2 receptors. They are able to remove intraneuronal Aβ, reduce oxidative damage, and protect from the loss of energy or trophic support. -

Potential Cannabis Antagonists for Marijuana Intoxication
Central Journal of Pharmacology & Clinical Toxicology Bringing Excellence in Open Access Review Article *Corresponding author Matthew Kagan, M.D., Cedars-Sinai Medical Center, 8730 Alden Drive, Los Angeles, CA 90048, USA, Tel: 310- Potential Cannabis Antagonists 423-3465; Fax: 310.423.8397; Email: Matthew.Kagan@ cshs.org Submitted: 11 October 2018 for Marijuana Intoxication Accepted: 23 October 2018 William W. Ishak, Jonathan Dang, Steven Clevenger, Shaina Published: 25 October 2018 Ganjian, Samantha Cohen, and Matthew Kagan* ISSN: 2333-7079 Cedars-Sinai Medical Center, USA Copyright © 2018 Kagan et al. Abstract OPEN ACCESS Keywords Cannabis use is on the rise leading to the need to address the medical, psychosocial, • Cannabis and economic effects of cannabis intoxication. While effective agents have not yet been • Cannabinoids implemented for the treatment of acute marijuana intoxication, a number of compounds • Antagonist continue to hold promise for treatment of cannabinoid intoxication. Potential therapeutic • Marijuana agents are reviewed with advantages and side effects. Three agents appear to merit • Intoxication further inquiry; most notably Cannabidiol with some evidence of antipsychotic activity • THC and in addition Virodhamine and Tetrahydrocannabivarin with a similar mixed receptor profile. Given the results of this research, continued development of agents acting on cannabinoid receptors with and without peripheral selectivity may lead to an effective treatment for acute cannabinoid intoxication. Much work still remains to develop strategies that will interrupt and reverse the effects of acute marijuana intoxication. ABBREVIATIONS Therapeutic uses of cannabis include chronic pain, loss of appetite, spasticity, and chemotherapy-associated nausea and CBD: Cannabidiol; CBG: Cannabigerol; THCV: vomiting [8]. Recreational cannabis use is on the rise with more Tetrahydrocannabivarin; THC: Tetrahydrocannabinol states approving its use and it is viewed as no different from INTRODUCTION recreational use of alcohol or tobacco [9]. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President,