Lecture # 5, 6 – Enzyme Inhibition and Toxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

The Self-Inhibitory Nature of Metabolic Networks and Its Alleviation Through Compartmentalization
ARTICLE Received 30 Oct 2016 | Accepted 23 May 2017 | Published 10 Jul 2017 DOI: 10.1038/ncomms16018 OPEN The self-inhibitory nature of metabolic networks and its alleviation through compartmentalization Mohammad Tauqeer Alam1,2, Viridiana Olin-Sandoval1,3, Anna Stincone1,w, Markus A. Keller1,4, Aleksej Zelezniak1,5,6, Ben F. Luisi1 & Markus Ralser1,5 Metabolites can inhibit the enzymes that generate them. To explore the general nature of metabolic self-inhibition, we surveyed enzymological data accrued from a century of experimentation and generated a genome-scale enzyme-inhibition network. Enzyme inhibition is often driven by essential metabolites, affects the majority of biochemical processes, and is executed by a structured network whose topological organization is reflecting chemical similarities that exist between metabolites. Most inhibitory interactions are competitive, emerge in the close neighbourhood of the inhibited enzymes, and result from structural similarities between substrate and inhibitors. Structural constraints also explain one-third of allosteric inhibitors, a finding rationalized by crystallographic analysis of allosterically inhibited L-lactate dehydrogenase. Our findings suggest that the primary cause of metabolic enzyme inhibition is not the evolution of regulatory metabolite–enzyme interactions, but a finite structural diversity prevalent within the metabolome. In eukaryotes, compartmentalization minimizes inevitable enzyme inhibition and alleviates constraints that self-inhibition places on metabolism. 1 Department of Biochemistry and Cambridge Systems Biology Centre, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK. 2 Division of Biomedical Sciences, Warwick Medical School, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. 3 Department of Food Science and Technology, Instituto Nacional de Ciencias Me´dicas y Nutricio´n Salvador Zubira´n, Vasco de Quiroga 15, Tlalpan, 14080 Mexico City, Mexico. -

NADPH-Dependent A-Oxidation of Unsaturated Fatty Acids With
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6673-6677, August 1992 Biochemistry NADPH-dependent a-oxidation of unsaturated fatty acids with double bonds extending from odd-numbered carbon atoms (5-enoyl-CoA/A3,A2-enoyl-CoA isomerase/A3',52'4-dienoyl-CoA isomerase/2,4-dienoyl-CoA reductase) TOR E. SMELAND*, MOHAMED NADA*, DEAN CUEBASt, AND HORST SCHULZ* *Department of Chemistry, City College of the City University of New York, New York, NY 10031; and tJoined Departments of Chemistry, Manhattan College/College of Mount Saint Vincent, Riverdale, NY 10471 Communicated by Salih J. Wakil, April 13, 1992 ABSTRACT The mitochondrial metabolism of 5-enoyl- a recent observation of Tserng and Jin (5) who reported that CoAs, which are formed during the (3-oxidation of unsaturated the mitochondrial -oxidation of 5-enoyl-CoAs is dependent fatty acids with double bonds extending from odd-numbered on NADPH. Their analysis of metabolites by gas chroma- carbon atoms, was studied with mitochondrial extracts and tography/mass spectrometry led them to propose that the purified enzymes of (3-oxidation. Metabolites were identified double bond of 5-enoyl-CoAs is reduced by NADPH to yield spectrophotometrically and by high performance liquid chro- the corresponding saturated fatty acyl-CoAs, which are then matography. 5-cis-Octenoyl-CoA, a putative metabolite of further degraded by P-oxidation. linolenic acid, was efficiently dehydrogenated by medium- This report addresses the question of how 5-enoyl-CoAs chain acyl-CoA dehydrogenase (EC 1.3.99.3) to 2-trans-5-cis- are chain-shortened by P-oxidation. We demonstrate that octadienoyl-CoA, which was isomerized to 3,5-octadienoyl- 5-enoyl-CoAs, after dehydrogenation to 2,5-dienoyl-CoAs, CoA either by mitochondrial A3,A2-enoyl-CoA isomerase (EC can be isomerized to 2,4-dienoyl-CoAs, which are reduced by 5.3.3.8) or by peroxisomal trifunctional enzyme. -

Plant Protease Inhibitors: a Defense Strategy in Plants
Biotechnology and Molecular Biology Review Vol. 2 (3), pp. 068-085, August 2007 Available online at http://www.academicjournals.org/BMBR ISSN 1538-2273 © 2007 Academic Journals Standard Review Plant protease inhibitors: a defense strategy in plants Huma Habib and Khalid Majid Fazili* Department of Biotechnology, The University of Kashmir, P/O Naseembagh, Hazratbal, Srinagar -190006, Jammu and Kashmir, India. Accepted 7 July, 2007 Proteases, though essentially indispensable to the maintenance and survival of their host organisms, can be potentially damaging when overexpressed or present in higher concentrations, and their activities need to be correctly regulated. An important means of regulation involves modulation of their activities through interaction with substances, mostly proteins, called protease inhibitors. Some insects and many of the phytopathogenic microorganisms secrete extracellular enzymes and, in particular, enzymes causing proteolytic digestion of proteins, which play important roles in pathogenesis. Plants, however, have also developed mechanisms to fight these pathogenic organisms. One important line of defense that plants have to fight these pathogens is through various inhibitors that act against these proteolytic enzymes. These inhibitors are thus active in endogenous as well as exogenous defense systems. Protease inhibitors active against different mechanistic classes of proteases have been classified into different families on the basis of significant sequence similarities and structural relationships. Specific protease inhibitors are currently being overexpressed in certain transgenic plants to protect them against invaders. The current knowledge about plant protease inhibitors, their structure and their role in plant defense is briefly reviewed. Key words: Proteases, enzymes, protease inhibitors, serpins, cystatins, pathogens, defense. Table of content 1. -

Effect of Statins and ACE Inhibitors Alone and in Combination on Clinical Outcome in Patients with Coronary Heart Disease
Journal of Human Hypertension (2004) 18, 781–788 & 2004 Nature Publishing Group All rights reserved 0950-9240/04 $30.00 www.nature.com/jhh ORIGINAL ARTICLE Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease VG Athyros1, DP Mikhailidis3, AA Papageorgiou2, VI Bouloukos1, AN Pehlivanidis1, AN Symeonidis4 and M Elisaf5, for the GREACE Study Collaborative Group 1Atherosclerosis Unit, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 2Department of Clinical Biochemistry, Royal Free Hospital, Royal Free and University College Medical School, Pond Street, London, UK; 32nd Propedeutic Department of Internal Medicine, Aristotelian University, Hippocration Hospital, Thessaloniki, Greece; 4Greek Society of General Practitioners, Thessaloniki, Greece; 5Department of Internal Medicine, Medical School, University of Ioannina, Greece We assessed the ‘synergy’ of statins and angiotensin- point in group A was 31%, (95% CI À48 to À6%, P ¼ 0.01) converting enzyme inhibitors (ACEI) in reducing vascu- in comparison to group B, 59% (95% CI À72 to À48%, lar events in patients with coronary heart disease (CHD). Po0.0001) to group C and 63% (95% CI À74 to À51%, The GREek Atorvastatin and CHD Evaluation (GREACE) Po0.0001) to group D. There was no significant Study, suggested that aggressive reduction of low difference in RRR between groups C and D (9%, CI density lipoprotein cholesterol to 2.59 mmol/l À27–10%, P ¼ 0.1). Other factors (eg the blood pressure) (o100 mg/dl) significantly reduces morbidity and mor- that can influence clinical outcome did not differ tality in CHD patients, in comparison to undertreated significantly between the four treatment groups. -

Enzyme2 File
Enzymes Theories of enzyme substrate interaction 2 Theories of enzyme substrate interaction . There are two proposed methods by which enzymes bind to their substrate molecules: 1- Lock & key model 2-Induced fit model 3 Theories of enzyme substrate interaction 1-lock & key model (Template hypothesis for enzyme action): The substrate fits to binding site of enzyme as a key fits into proper lock . Enzyme & substrate possess specific complementary geometric & rigid shapes fit exactly into one another. This explains enzyme specificity, but fails to explain the stabilization of the transition state & allosteric regulation & inhibition. 4 1-lock & key model : 5 Theories of enzyme substrate interaction 2-Induce fit model More flexible model than lock & key model. Enzymes are flexible structures The active site modified as the [S] interacts with the anzyme (active site of enzyme become complementary to S) Model explains enzyme specificty & stabilization of transition state, regulation & inhibition. 6 2-Induce fit model 7 Enzyme Inhibition 8 Enzyme inhibitors: Any substance that can diminish the velocity of an enzyme-catalyzed reaction. 9 Types of inhibition Irreversible Reversible inhibitors inhibitors 10 Types of inhibition 1-Irreversible inhibitors : Bind to enzymes through covalent bond (inhibited enzyme does not regain activity upon dilution of enzyme-inhibitor complex) 11 Type of inhibition 2-Reversible inhibitors: Bind to enzymes through noncovalent bonds, thus dilution of the enzyme–inhibitor complex results in dissociation of the reversibly bound inhibitor, and recovery of enzyme activity. 12 Reversible inhibitors 1. Competitive inhibition 2. Non competitive inhibition 3. Uncompeptitive inhibition 13 1-Competitive inhibition The inhibitor and substrate compete for the same active site on the enzyme as a result of similarity in structure. -

Chapter 7. "Coenzymes and Vitamins" Reading Assignment
Chapter 7. "Coenzymes and Vitamins" Reading Assignment: pp. 192-202, 207-208, 212-214 Problem Assignment: 3, 4, & 7 I. Introduction Many complex metabolic reactions cannot be carried out using only the chemical mechanisms available to the side-chains of the 20 standard amino acids. To perform these reactions, enzymes must rely on other chemical species known broadly as cofactors that bind to the active site and assist in the reaction mechanism. An enzyme lacking its cofactor is referred to as an apoenzyme whereas the enzyme with its cofactor is referred to as a holoenzyme. Cofactors are subdivided into essential ions and organic molecules known as coenzymes (Fig. 7.1). Essential ions, commonly metal ions, may participate in substrate binding or directly in the catalytic mechanism. Coenzymes typically act as group transfer agents, carrying electrons and chemical groups such as acyl groups, methyl groups, etc., depending on the coenzyme. Many of the coenzymes are derived from vitamins which are essential for metabolism, growth, and development. We will use this chapter to introduce all of the vitamins and coenzymes. In a few cases--NAD+, FAD, coenzyme A--the mechanisms of action will be covered. For the remainder of the water-soluble vitamins, discussion of function will be delayed until we encounter them in metabolism. We also will discuss the biochemistry of the fat-soluble vitamins here. II. Inorganic cation cofactors Many enzymes require metal cations for activity. Metal-activated enzymes require or are stimulated by cations such as K+, Ca2+, or Mg2+. Often the metal ion is not tightly bound and may even be carried into the active site attached to a substrate, as occurs in the case of kinases whose actual substrate is a magnesium-ATP complex. -
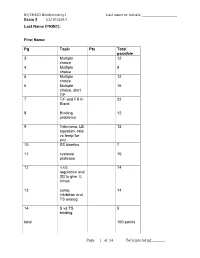
BI/CH421 Biochemistry I Last Name Or Initials: ______Exam 3 11/10/2014 Last Name (PRINT)
BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Last Name (PRINT): First Name: Pg Topic Pts Total possible 3 Multiple 12 choice 4 Multiple 9 choice 5 Multiple 12 choice 6 Multiple 16 choice, start T/F 7 T/F and Fill in 22 Blank 8 Binding 12 problems 9 Yakimima, LB 12 equation, rate vs temp for enz 10 SS kinetics 7 11 cysteine 15 protease 12 ∆∆G, 14 regulation and [S] to give ¾ Vmax 13 comp. 14 inhibition and TS analog 14 S vs TS 5 binding total 150 points Page of 11 4 Total pts for pg _________ BI/CH421 Biochemistry I Last name or initials: ________________________ Exam 3 11/10/2014 Instructions: READ INSTRUCTIONS BEFORE BEGINNING EXAM. 1) Carefully read question before answering. Often I highlight very important information so please make note when I do so to make sure you are answering the question correctly. 2) Write your FULL name above and at least your last name or initials on every page. 3) Write all of your answers on the exam paper itself in the space provided. If you need additional space, you can write on the back of the SAME page. If you do this, you must write “ON BACK” so that we know where to look for your answer. 4) Your answers should be brief and legible. A correct answer that cannot be read cannot receive full credit. Additionally, extremely lengthy responses containing both correct and incorrect statements will be graded accordingly. Meaning, if you answer the question correctly but if you go on to write a “kitchen sink” response containing incorrect information, you will not receive full credit for that answer. -

Enzymes Main Concepts: •Proteins Are Polymers Made up of Amino Acids
Biology 102 Karen Bledsoe, Instructor Notes http://www.wou.edu/~bledsoek/ Chapter 6, section 4 Topic: Enzymes Main concepts: • Proteins are polymers made up of amino acids. Enzymes are a category of proteins. • Enzymes are catalysts. They speed up the rate of a chemical reaction by reducing the activation energy, which is the energy needed to carry out the reaction. An example of a catalyst: if you put a lit match to a sugar cube, it’s hard to make the sugar catch fire. But if you rub a corner of the sugar cube with ash or charcoal, it catches more easily. The ash or charcoal acts as a catalyst. • Many important chemical reactions in living cells can happen spontaneously, but these reactions happen too rarely and too slowly to sustain life. Enzymes make the reactions happen more quickly and more often. • Some reactions happen too violently to support life. Sugar, when burned, released a lot of energy, but the energy is mostly heat. A rapid reaction like that would overheat our cells. Enzyme-controlled reactions can release energy from sugar in a way that captures most of the energy and loses less of the energy as heat, so that the energy is useful to the cell and does not overheat the cell. • The structure of enzymes allows them to carry out reactions. Each enzyme is shaped to carry out only one specific reaction. This is known as enzyme specificity. Amylase, for example, is an enzyme that breaks down starch into glucose. Amylase only breaks down starch; it does not break down any other molecule, nor does it build starch out of glucose. -

Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice
pharmaceutics Review Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice Malavika Deodhar 1, Sweilem B Al Rihani 1 , Meghan J. Arwood 1, Lucy Darakjian 1, Pamela Dow 1 , Jacques Turgeon 1,2 and Veronique Michaud 1,2,* 1 Tabula Rasa HealthCare Precision Pharmacotherapy Research and Development Institute, Orlando, FL 32827, USA; [email protected] (M.D.); [email protected] (S.B.A.R.); [email protected] (M.J.A.); [email protected] (L.D.); [email protected] (P.D.); [email protected] (J.T.) 2 Faculty of Pharmacy, Université de Montréal, Montreal, QC H3C 3J7, Canada * Correspondence: [email protected]; Tel.: +1-856-938-8697 Received: 5 August 2020; Accepted: 31 August 2020; Published: 4 September 2020 Abstract: In an ageing society, polypharmacy has become a major public health and economic issue. Overuse of medications, especially in patients with chronic diseases, carries major health risks. One common consequence of polypharmacy is the increased emergence of adverse drug events, mainly from drug–drug interactions. The majority of currently available drugs are metabolized by CYP450 enzymes. Interactions due to shared CYP450-mediated metabolic pathways for two or more drugs are frequent, especially through reversible or irreversible CYP450 inhibition. The magnitude of these interactions depends on several factors, including varying affinity and concentration of substrates, time delay between the administration of the drugs, and mechanisms of CYP450 inhibition. Various types of CYP450 inhibition (competitive, non-competitive, mechanism-based) have been observed clinically, and interactions of these types require a distinct clinical management strategy. This review focuses on mechanism-based inhibition, which occurs when a substrate forms a reactive intermediate, creating a stable enzyme–intermediate complex that irreversibly reduces enzyme activity. -

Digestive Enzyme: Amylase
DIGESTIVE ENZYME: AMYLASE BACKGROUND Caution: Most animals begin their digestion in their mouths. Chewing breaks up Lugol’s solution is slightly large pieces of food and chemicals in the saliva begin breaking apart hazardous: molecules of starch. In this experiment we add saliva to crackers to • It should not be observe the how quickly this process begins to happen. handled by small children. Basic definitions: • It is dangerous if Amylase: an enzyme that breaks down starch into sugars. swallowed. Enzyme: a protein that speeds up a chemical reaction. • Participants should Chemical Indicator: a chemical that allows you to observe that a wear gloves. reaction has taken place. • Adults should place Starch: a complex arrangement of sugar molecules. the Lugol’s solution into a small dropper Starch is a long group of glucose (sugar) molecules bonded together. bottle for youth to Amylose is the starch form that’s found in crackers. In amylose, the use. sugar molecules form a structure that has small spaces in between the molecules of sugar. Lugol’s solution contains iodine molecules that fit Skills: tightly inside these small spaces. When the iodine molecules are inside • Observation, these small spaces between bonded sugar molecules, the iodine looks teamwork. blue-black in color. If the sugar molecules begin to break apart and release the iodine molecules, the indicator solution looks light brown- Grade Levels: Grades 4 - brown in color. 12 Amylase is the protein that breaks apart starch into sugars. Amylase is a • Time: 15 – 20 minutes chemical found in the saliva of many animals. Number of Youth: Materials for each test group: Any Lugol’s Solution (2%) in small dropper bottle 2 test tubes with lids : 25ml or 50 ml Supplies Needed: Small cup of water Materials for each test Crushed saltine cracker in zip lock bag group: 5-7 plastic cups: 3 oz. -

Study Protocol
Full title: A Sequential Phase I study of MEK1/2 inhibitors PD-0325901 or Binimetinib combined with cMET inhibitor PF-02341066 in Patients with RAS Mutant and RAS Wild Type (with aberrant c-MET) Colorectal Cancer Short title: MErCuRIC1: MEK and MET Inhibition in Colorectal Cancer Protocol Version & date: Version 9.0; 29Oct2018 Sponsor Protocol Number: OCTO_049 Ethics Number: 14/SC/1010 EudraCT Number: 2014-000463-40 Funding Reference Number 602901-2 Sponsored by the University of Oxford Funder: European Commission’s Seventh Framework Program (FP7) Confidentiality Statement This document contains confidential information that must not be disclosed to anyone other than the Sponsor, the Trials Office, the Investigator Team, host NHS Trust(s), regulatory authorities, and members of the Research Ethics Committee. MErCuRIC1_Protocol_V9.0_29Oct2018 Protocol Template V3.0_18Feb2013 Page 1 of 121 MErCuRIC1 Confidential GENERAL CONTACT INFORMATION Trial Office (OCTO) MErCuRIC Trial Office Oncology Clinical Trials Office (OCTO) Department of Oncology, The University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford Tel: +44 (0)1865 227194 Email: [email protected] Website: http://www.oncology.ox.ac.uk/research/oncology- clinical-trials-office-octo Chief Investigator Professor Mark Middleton Department of Oncology, University of Oxford Oxford Cancer and Haematology Centre Churchill Hospital Oxford OX3 7LE Tel: +44 (0)1865 235 315 Email : [email protected] Investigator Professor Richard Wilson and