Carly Thesis Final.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
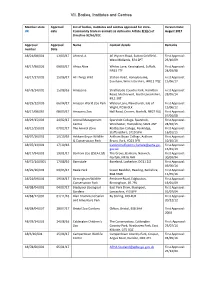
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

ATIC0943 {By Email}
Animal and Plant Health Agency T 0208 2257636 Access to Information Team F 01932 357608 Weybourne Building Ground Floor Woodham Lane www.gov.uk/apha New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC0943 {By Email} 4 October 2016 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about zoos which we received on 26 September 2016. Your request has been handled under the Freedom of Information Act 2000. The information you requested and our response is detailed below: “Please can you provide me with a full list of the names of all Zoos in the UK. Under the classification of 'Zoos' I am including any place where a member of the public can visit or observe captive animals: zoological parks, centres or gardens; aquariums, oceanariums or aquatic attractions; wildlife centres; butterfly farms; petting farms or petting zoos. “Please also provide me the date of when each zoo has received its license under the Zoo License act 1981.” See Appendix 1 for a list that APHA hold on current licensed zoos affected by the Zoo License Act 1981 in Great Britain (England, Scotland and Wales), as at 26 September 2016 (date of request). The information relating to Northern Ireland is not held by APHA. Any potential information maybe held with the Department of Agriculture, Environment and Rural Affairs Northern Ireland (DAERA-NI). Where there are blanks on the zoo license start date that means the information you have requested is not held by APHA. Please note that the Local Authorities’ Trading Standard departments are responsible for administering and issuing zoo licensing under the Zoo Licensing Act 1981. -

Annual Report
Annual Report 2018 - 19 Contents Page Patrons and Presidents 2 Glossary 3 Foreword 4 1. Scope and format of report 5 2. National Programme 5 3. Recruiting and Retaining Members 7 4. Supporting Volunteers 8 5. Building Sponsorship 8 6. Managing the Business 9 7. Building the Business 10 Appendices 1. National and Regional Management Committees 13 2. 2018/2019 National HASSRA Decision-Making Bodies 14 3. Report from Awards Committee Chair 15 4. Report from Executive Committee Chair 16 5. Report from Finance Committee Chair 17 6. 2018 HASSRA Festival Results 18 7. Membership Benefits 20 8. HASSRA Membership 29 9. 2018 Finance Transactions 33 10. 2018 HASSRA Website Hits 34 11. 2018 Member Survey Results 35 1 Patrons The Rt. Hon. Amber Rudd MP, Secretary of State for Work and Pensions The Rt. Hon. Matt Hancock MP, Secretary of State for Health and Social Care Alok Sharma MP, Minister of State for Employment Stephen Hammond MP, Minister of State for Health Caroline Dinenage MP, Minister of State for Care Sarah Newton MP, Minister of State for Disabled People, Health and Work Justin Tomlinson MP, Parliamentary Under Secretary of State for Work and Pensions Steve Brine MP, Jackie Doyle-Price MP and Baroness Blackwood, Parliamentary Under Secretaries of State for Health President Peter Schofield CB, Permanent Secretary, Department for Work and Pensions First Vice President Sir Chris Wormald KCB, Permanent Secretary, Department of Health and Social Care Vice Presidents DWP Susan Park Director General Operations John-Paul Marks Director General Universal Credit Operations DH Prof. Dame Sally Davies Chief Medical Officer Dr Ian Hudson Chief Exec. -

2021-Escorted-Tours-Family-Fun-Brochure-Compressed.Pdf
Local Travel Solutions Low Deposit Transfers Award-Winning Departure Points Representative From £50pp Included Tours www.travel-solutions.co.uk As Northern Ireland's leading tour operator, we take the time to source itineraries that create memorable experiences for our customers. For Escorted Coach Tours throughout the UK & Ireland, Family Fun Adventures or a week in the beautiful island of Jersey, we take care of all the planning and organisation so you don't have to. We are proud of our awarding winning team, not only were we crowned 'Best Escorted Tour Operator in 2018, but again in 2019 at the prestigious Northern Ireland Travel & Tourism Awards. But, we never rest on our laurels as we understand that we must work harder to maintain the high standards and great value that we have become renowned for. We have been operating escorted tours for twenty five years and our team works hard to ensure we get a great mix of our most popular tours together with tours to whet your appetite for something new. Above all, you can book with confidence. Our award winning services, passion for travel and attention to detail to our tours will bring a whole new experience to your holiday. So what are you waiting for? Take a look inside our brochure, packed full of tours designed with you in mind. A big thank you to those many customers who travel with us year after year. We are always pleased to welcome you back, and we are also excited to welcome new customers, as this allows us to showcase our tours. -

Quarter 2, 2014 Dates for Your Diary Guess Who Came to Play at Web Adventure Park! Zero Hour Contracts Update
In this issue: BALPPA does Barcelona FEC Seminar at LEGOLAND – May 2014 Quarter 2, 2014 Dates for your diary Guess who came to play at Web Adventure Park! Zero Hour Contracts update SUMMER’S HERE! Quarter 2 of 2014 has flown by! Along with various seminars and sub group meetings, we also held the 2014 Summer Conference in Barcelona which was a great success. BALPPA DOES BARCELONA We have recently returned from the Summer Conference and thoroughly enjoyed it! This year’s conference took place in Barcelona, Spain and our guests enjoyed a golf day, city tour, visit to Port Aventura, our Gala Dinner with stunning 360 degree views across the city as well as flamenco dancers. We also visited Tibidabo theme park where we were treated to more stunning views of Barcelona some great attractions and BALPPA CEO, Paul Kelly a sumptuous lunch. enjoys the stunning surroundings at Port Thank you to everyone who came along – we hope you Aventura against a enjoyed it! Thanks also to our very generous sponsors who all rather unfortunate back received awards, presented by BALPPA Chairman, Bob drop! O’Connor at the Gala Dinner: Steven Wooley, Embed Phil Pickersgill, Derek Smart, The Noisy Karen Staley, IAAPA Innovative Leisure Drinks Company Europe INNOVATIVE LEISURE GIVES TWO LOCATIONS THE SKY TRAIL TREATMENT. Innovative Leisure has installed High Ropes attractions at two UK locations - the National Water Sports Centre (NWSC) in Nottingham and North Bay Railway in Scarborough. The NWSC has installed a double level Navigator course which pits its adventurous clientèle against 36 hair-raising rope elements at heights of up to 10m. -

Our Great SSA Offers for 2014 Are Now Available, If You
All our great SSA Offers for 2014 are now available, if you are interested in any of the offers please contact the Central Veterans Office on 020 7695 7190 or 020 7695 4934 SSA offers include: Great Days Out – Include NEW FOR 2014 Dublin Zoo – up to £3.00 per ticket saving Chester Zoo – Discounted rate depends on date of visit please call for with details and we will confirm availability and price Woburn Abbey – up to £4.00 per ticket saving Flamingo Land – up to £12 .00 per ticket saving Great Getaways include: Hoseasons, Cottages 4 U, Pontins, Haven, Park Resorts Warner Leisure Hotels, Potters Leisure Resort, Old English Inns, Holidays in Cornwall, High Hagg Cottages, Airport Parking, IHG Hotels Save up to 15% with Airport Parking & Hotels Irish Ferries 2014 – Save 10% on holiday packages 1 2014 Great Days Out! Colchester Zoo, Essex Savings of up to £5.00 in Peak season Paultons Family Theme Park, The New Forest, Hants Savings of up to £2.00 Adventure Island, Southend, Essex Savings of up to £12 West Midland Safari Park, Worcestershire Savings of up to 35% Dublin Zoo, Landmark Park & Woburn Safari Park NEW 2014 savings of up to £3.00 Savings up to £4.00 Landmark Forest Adventure Park Scotland 10% off 2 2014 Merlin Theme Parks 2014 The Alton Towers Resort Chessington World of Adventures Resort Save up to 40% Thorpe Park Save up to 56% Legoland® Windsor Resort Save up to 40% Madame Tussauds London Save up to 25% 3 2014 Warwick Castle Save up to 40% Edinburgh Dungeon Save up to 25% and 40% on York Dungeons Save up to 25% Save up to 40% Includes Brighton, Birmingham, Manchester and more…. -

HASSRA W.M. NEWS July 2021
HASSRA W.M. NEWS July 2021 HASSRA National Lottery Winners: Congratulations to the latest West Midlands HASSRA national lottery winners in the June 2021 draw: £1,000: Azmina Mirza (Nuneaton Debt Centre) £500: Anonymous winner (Birmingham Five Ways House) £500: Anonymous winner (Birmingham Centennial House) £500: Louise Fletcher (Coventry Cofa Court) £500: Anonymous winner (Birmingham Five Ways House) £250: Anonymous winner (Halesowen JCP) £100: Anonymous winner (Wolverhampton Molineux House) £100: Tracey Powell (Stourbridge JCP) £100: Stephanie Wiggett (Solihull JCP) £100: Roger Price (Shrewsbury JCP) You can view the latest lottery winners in full at www.hassra.org.uk/lottery-results Euro 2020 Competition Results A total of 462 members entered the Euro 2020 competition, of which 336 answered correctly. With Wembley Stadium hosting several of the Euro 2020 fixtures, the manager of England the last time they hosted a tournament in Euro ’96 was Terry Venables. Congratulations to our 3 winners drawn from HASSRA Live: Position Prize Winner Winner £40 Love2Shop e-code Claire McDermott (Nuneaton Debt Centre) £10 Love2Shop e-code Helen Clee (Telford Newtown House) Runner Up £10 Love2Shop e-code Debra Davies (Halesowen JCP) Warwick Castle Cashback Offer Visit Warwick Castle between 1st - 31st August 2021 and get 25% cashback on your ticket purchases courtesy of HASSRA West Midlands. A great day out, with great savings from HASSRA! Simply purchase your discounted tickets through HASSRA Live and claim your cashback after your visit! Terms & Conditions: 25% cashback value is up to a maximum of x4 One Day Entry Peak Tickets purchased (HASSRA price of £20 each). Email your booking confirmation / proof of purchase, along with the fully completed cashback proforma to [email protected]. -

Rainbows Go W D I L
Rainbows Go W D I L Information pack April 2019 What is Rainbows Go Wild? Rainbows Go Wild is your Rainbow unit visiting a zoo, wildlife park or aquarium to find out more about the animals found there. We are also encouraging you to hold a sleepover, perhaps with your own wildlife theme! We want as many Rainbow units across Girlguiding LaSER as possible to take part in Rainbows Go Wild. In July, we will be sending out more information in the activity pack, which will also contain all the activities, advice and ideas you'll need to make this event happen for your Rainbows. This information pack includes an FAQ section with all the important information you'll need to know. We also have the details of the 21 venues (listed below) for you to pick the one your unit will go wild at! If there's something we haven't thought of, get in touch on [email protected]. Please read through this information pack and register your interest here. Where will your Rainbows go wild? Battersea Park Children's Zoo Kent Life Bird World Merrist Wood British Wildlife Centre Paradise Wildlife Park Chessington World of Adventures Port Lympne Drusillas Park Sealife London Aquarium Eagle Heights Tilgate Nature Centre Fisher's Farm Park Wildwood Trust Hanwell Zoo Wingham Wildlife Park Hobbledown WWT Arundel Howletts WWT London Wetland Centre ZSL London Zoo Venues and prices Venue Battersea Park Children’s Zoo, Chelsea Bridge Gate, Battersea, London, SW11 4NJ Rainbows Go Wild Child: £6.50 Price Adult: Free accompanying adult per 5 children (additional adults £9.50). -

Licensing - WK/201404120
Licensing - WK/201404120 Property Address Paradise Wildlife Park, White Stubbs Lane, Bayford, Hertfordshire, EN10 7QA Telephone 01992 470490 Fax Area Worksheet Source Name Address Paradise Wildlife Park, White Stubbs Lane, Bayford, Hertfordshire, EN10 7QA Home Tel Work Tel Mobile Fax01992 440525 Email Department EH - Environmental HealthDate 03/06/2014 Time 11:25 Application LN - Licensing Method A - Application Task Group LPR - Premises Recd By Task L05 - Zoo Source Type Details Details found on WK/201400692 Message Officer Target Actual Time Taken 0 First Response Next Task Completed 21/01/2015 Due Allocated 26/08/2014 Outcome L002 - Licence Reissued References No references set up. Licensing Transaction 2 - Renewal Reg. Name Parkside Leisure Limited Application 26/04/2014 Targets New period Premises Band Application Complete 26/08/2014 Start 01/09/2014 Representations By Period 72 Months Fee Information Issue 26/08/2014 Expiry 31/08/2020 Standard 1,513.00 Other 3,592.15 Existing Licence if present Total £5,105.15 Start 01/09/2008 Receipt DILI0008475 Expiry 31/08/2014 Date Paid 09/01/2015 Licence closed and not issued Status Renewal Issued Date Reason Premises No Premises data found. Pre-Requisites Pre-Requisite VET1 - Recoverable Vet Fee 1 Valid / Check Date 26/08/2014 Expiry Date Notes £946.96 + £190 VAT Officer Public Protection by Northgate Page 1 of 3 Licensing - WK/201404120 Value£ 1,136.96 Time Pre-Requisite VET2 - Recoverable Vet Fee 2 Valid / Check Date 26/08/2014 Expiry Date Notes Officer Value£ 1,266.79 Time Pre-Requisite VET3 - Recoverable Vet Fee 3 Valid / Check Date 26/08/2014 Expiry Date Notes Officer Value£ 1,188.40 Time Operating Schedule No Operating Schedule found. -

UK-First-Breeding-Register.Pdf
INTRODUCTION TO EDITION The original author and compiler of this record, Dave Coles has devoted a great deal of his time in creating a unique reference document which I am privileged to continue working with. By its very nature the document in a living project which will continue to grow and evolve and I can only hope and wish that I have the dedication as Dave has had in ensuring the continuity of the “First Breeding Register”. Initially all my additions and edits will be indicated in italics though this will not be used in the main body of the record (when a new record is added). Simon Matthews (2011) It is over eighty years since Emilius Hopkinson collated his RECORDS OF BIRDS BRED IN CAPTIVITY, which formed the starting point for my attempt at recording first breeding records for the UK. Since the first edition of my records was published in 1986, classification has changed several times and the present edition incorporates this up-dated classification as well as first breeding recorded since then. The research needed to produce the original volume took many years to complete and covered most avicultural literature. Since that time numerous people have kept me abreast of further breedings and pointing out those that have slipped the net. This has hopefully enabled the records to be kept up-to- date. To those I would express my thanks as indeed I do to all that have cast their eyes over the new list and passed comments, especially the ever helpful Reuben Girling who continues to pass on enquiries. -

Amazing Amphibians Celebrating a Decade of Amphibian Conservation
QUARTERLY PUBLICATION OF THE EUROPEAN ASSOCIATION OF ZOOS AND AQUARIA AUTUMNZ 2018OO QUARIAISSUE 102 AMAZING AMPHIBIANS CELEBRATING A DECADE OF AMPHIBIAN CONSERVATION A giant challenge BUILDING A FUTURE FOR THE CHINESE GIANT SALAMANDER 1 Taking a Leap PROTECTING DARWIN’S FROG IN CHILE Give your visitors a digital experience Add a new dimension to your visitor experience with the Aratag app – for museums, parks and tourist www.aratag.com attractions of all kinds. Aratag is a fully-integrated information system featuring a CMS and universal app that visitors download to their smart devices. The app runs automatically when it detects a nearby facility using the Aratag system. With the power of Aratag’s underlying client CMS system, zoos, aquariums, museums and other tourist attractions can craft customized, site-specifi c app content for their visitors. Aratag’s CMS software makes it easy for you to create and update customized app content, including menus, text, videos, AR, and active links. Aratag gives you the power to intelligently monitor visitors, including demographics and visitor fl ows, visit durations, preferred attractions, and more. You can also send push messages through the app, giving your visitors valuable information such as feeding times, closing time notices, transport information, fi re alarms, evacuation routes, lost and found, etc. Contact Pangea Rocks for an on-site demonstration of how Aratag gives you the power to deliver enhanced visitor experiences. Contact us for more information: Address: Aratag is designed and Email: [email protected] Aratag / Pangea Rocks A/S developed by Pangea Rocks A/S Phone: +45 60 94 34 32 Navervej 13 in collaboration with Aalborg Mobile : +45 53 80 34 32 6800 Varde, Denmark University.