Heart-Lung Interactions: Implications for Non-Invasive Evaluation of Changes in Blood Volume
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Passive Leg Raising Towards Hemodynamics on Patient with Hypovolemic Shock at the Emergency Ward of Dustira Cimahi Hospital
International Seminar on Global Health (ISGH) 2017 Stikes Jenderal Achmad Yani Cimahi The Effect of Passive Leg Raising towards Hemodynamics on Patient with Hypovolemic Shock at the Emergency Ward of Dustira Cimahi Hospital Evangeline Hutabarat Nursing Science Dept., Stikes Jenderal Achmad Yani Cimahi Email: [email protected] Abstract One of the conditions needing immediate response in the emergency ward is hypovolemic shock. The patient in shock requires close monitoring for clinical signs and hemodynamic status and intravascular status. It is due to circulatory assistance and medication provision on the patient is given based on the accuracy of the intravascular volume status of the patient. Fluid responsiveness can be considered as basis for considering administering fluid only or inotropic and vasopressor medication for the patient to maintain her circulatory homeostatic. Passive Leg Raising (PLR) may be a reversible method to evaluate fluid responsiveness. The study aims at evaluating the effect of PLR on hemodynamic. It employs the quasi-experimental design with the within subject repeated measurement design approach. 48 respondents took part as samples, with the consecutive sampling method implemented. The samples were then categorized into responsive and non-responsive groups based on the increase of pulse pressure amounting to or beyond 9% during PLR implementation. The data was tested using the dependent and independent t test to provide analysis for uni-variate and bivariate data. The results indicated that 34 respondents belonged to the responsive group while 14 respondents were categorized as non-responsive. There was a significant relationship between PLR and hemodynamic parameters of systolic blood pressure, diastolic blood pressure, mean arterial pressure, and pulse pressure (p>0.05). -

100% NON-INVASIVE FLUID MANAGEMENT Proper Fluid Management May Improve Clinical Outcomes, Potentially Saving Millions in Operating Costs1,2
100% NON-INVASIVE FLUID MANAGEMENT Proper Fluid Management May Improve Clinical Outcomes, Potentially Saving Millions in Operating Costs1,2 The Starling fluid management monitoring system provides a full hemodynamic profile within seconds. The effect of fluids can be monitored at any time and treatment modified accordingly, across the continuum of care: ED > ICU > OR > RRT In a retrospective, matched, single-center study of nearly 200 patients, researchers from the University of Kansas Health System evaluated stroke volume (SV) guided resuscitation in 100 ICU patients with severe sepsis and septic shock and found1: Initiation of Acute % ICU Length of Stay -2.89 DAYS Dialysis Therapy -13.25 Save an estimated Risk of Mechanical % $14,498 per Ventilation -51 treated patient3 $14K Starling - Over 80% of hospitalized patients receive IV fluids.4 Yet studies show that giving too little or too much fluid can lead to serious complications and contribute to rising healthcare costs.5,6 - Studies also show that only ~50% of hemodynamically unstable patients will respond to IV fluid by increasing cardiac output and perfusion. Assessing whether fluid may help or harm a patient is a critical step in optimizing treatment.7 - Using only blood pressure, urine output and heart rate to measure fluid responsiveness may provide limited and inconclusive information.7 TAKE THE GUESSWORK OUT OF YOUR FLUID ASSESSMENT WITH STARLING - Provides a dynamic assessment of fluid responsiveness — accurately, precisely and 100% non-invasively. - Supports individualized fluid therapy without requiring an invasive arterial or central line, potentially reducing the risk of hospital-acquired infections and other complications.8 - Independently validated vs. -

Determination of Cardiac Output by Equating Venous Return Curves with Cardiac Response Curves
Determination of Cardiac Output By Equating Ve- nous Return Curves With Cardiac Response Curves1 ARTHUR C. GUYTQN From the Department of Physiology and Biophysics, School of Medicine, University of Mississippi, University, Mississippi HE CONCEPT that the heart responds with increasing cardiac output when there occurs increasing venous return was popularized by Starling and, in- deed, has come to be known as Starling’s law. There are many different forms in which Starling’s law can be expressed,including the relationship of cardiac output to right atria1 pressure, the relationship of cardiac output to the degree of distention of the right ventricle at the end of diastole, the relationship of cardiac work to right atria1 pressure or right ventricular distention, the relationship of left ventricular work to right atria1 pressure or right ventricular distention, etc. For the determina- tion of cardiac output, the form of Starling’s law which will be used in the present discussion is the relationship of cardiac output to mean right atria1 pressure, and this type of cruve will be called the “cardiac responsecurve” to right atria1 pressure. It is well known that many factors in the peripheral circulatory system combine together to determine the rate of venous return to the heart. These include the quantity of blood available, the degree of vascular resistance in various parts of the peripheral circulatory system, and the back pressure from the right atrium. It is with these factors that this paper is especially concerned, and it is hoped that this presentation will demonstrate how cardiac output is determined by equating the peripheral circulatory factors with the cardiac responsecurves. -
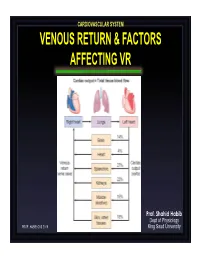
Venous Return & Factors Affecting Vr
CARDIOVASCULAR SYSTEM VENOUS RETURN & FACTORS AFFECTING VR Prof. Shahid Habib Dept of Physiology PROF. HABIB CVS 2019 King Saud University OBJECTIVES At the end of the lecture you should be able to ….. Discuss functions of the veins as blood reservoirs. Describe measurement of central venous pressure (CVP) and state its physiological and clinical significance. State determinants of venous return and explain how they influence venous return. Define mean systemic filling pressure, give its normal value and describe the factors which affect it. Explain the effect of gravity on venous pressure and explain pathophysiology of varicose veins. Describe vascular and cardiac function curves under physiological and pathophysiological conditions. DISTRIBUTION OF BLOOD Capacitance Vessels Veins are blood reservoirs When the body is at rest and many of the capillaries are closed, the capacity of the venous reservoir is increased as extra blood bypasses the capillaries and enters the veins. When this extra volume of blood stretches the veins, the blood moves forward through the veins more slowly because the total cross sectional area of the veins has increased as a result of the stretching. Therefore, blood spends more time in the veins. When the stored blood is needed, such as during exercise, extrinsic factors reduce the capacity of the venous reservoir and drive the extra blood from the veins to the heart so that it can be pumped to the tissues. Rt. Lt. At At Right Left ven ven Large veins Large arteries CAPACITANCE VESSELS EXCHANGE VESSELS RESISTANCE VESSELS Venules Capillaries Arterioles Vascular circuit • all 3 layers are present, but thinner than in •In varicose veins, arteries of corresponding size (external blood pools because diameter). -

Passive Leg Raise Testing Effectively Reduces Fluid Administration In
Rameau et al. Ann. Intensive Care (2017) 7:2 DOI 10.1186/s13613-016-0225-6 RESEARCH Open Access Passive leg raise testing effectively reduces fluid administration in septic shock after correction of non‑compliance to test results Arjanne Rameau, Eldert de With and Evert Christiaan Boerma* Abstract Background: Fluid resuscitation is considered a cornerstone of shock treatment, but recent data have underlined the potential hazards of fluid overload. The passive leg raise (PLR) test has been introduced as one of many strategies to predict ‘fluid responsiveness.’ The use of PLR testing is applicable to a wide range of clinical situations and has the potential to reduce fluid administration, since PLR testing is based upon (reversible) autotransfusion. Despite these theoretical advantages, data on the net effect on fluid balance as a result of PLR testing remain scarce. Methods: We performed a prospective single-center multi-step interventional study in patients with septic shock to evaluate the effect of implementation of PLR testing on the fluid balance (FB) 48 hours after ICU admission. All patients were equipped with a PiCCO® device for pulse contour analysis to guide fluid administration. An increase in stroke volume (SV) 10% was considered a positive test result. ≥ Results: Before introduction of PLR testing, 21 patients were prospectively included in period 1 with a median FB of 4.8 [3.3–7.8]L. After an extensive training program, PLR testing was introduced and 20 patients were included in period 2. Median FB was 4.4 [3.3–7.5]L and did not differ from period 1 (p 0.72). -

The Use of Passive Leg Raise Test to Predict Hypotension in Patients Undergoing Urological Procedures Under Spinal Anesthesia
Abdullah MW, et al., J Anesth Clin Care 2017, 4: 021 DOI: 10.24966/ACC-8879/100021 HSOA Journal of Anesthesia and Clinical Care Clinical Perspective these outcomes were not related to an individual’s cardiac output The Use of Passive Leg Raise response to the PLR test before spinal anesthesia. Conclusion: The use of PLR test to predict the occurrence of hy- Test to Predict Hypotension in potension in patients undergoing urological procedures under spinal anesthesia was not useful. Patients Undergoing Urolog- Keywords: Passive leg raise; Spinal anesthesia; Spinal hypoten- ical Procedures under Spinal sion; Urologic procedures Introduction Anesthesia Transurethral surgeries are common surgical procedures that can Mai Wedad Abdullah, Mona Hossam EL Din Abdelhamid*, Nor- be performed under general or spinal anesthesia [1], spinal anesthesia han Abdel Aleim Ali and Atef Galal Abdel Moula has proved to be an excellent choice for such surgeries [2]. Hypoten- sion after spinal anesthesia remains a common and potentially seri- Department of Anesthesia and Pain Management, Faculty of Medicine, Cairo University, Egypt ous complication, despite the use of prophylactic ephedrine and fluid loading with crystalloids [3]. Hypotension during spinal anesthesia is due to sympathetic blockade which leads to vasodilatation with a reduction of systemic vascular resistance. Extensive block may lead to decreased venous return which is the main determinant of hypoten- Abstract sion during central neuraxial blockade [4]. Aim of study: A prospective observational study to examine wheth- Most patients scheduled for endoscopic urological surgery are el- er Passive Leg Raise Test (PLR) administered with non invasive car- derly and frequently suffered from cardiopulmonary, endocrine, or diac output monitoring by the electrical Cardiometry could be used other co morbidities [5]. -

Comparison of the Accuracy of Monitoring Devices in Prediction of Fluid Responsiveness in Severe Sepsis and Septic Shock
IRB# 15-21 COMPARISON OF THE ACCURACY OF MONITORING DEVICES IN PREDICTION OF FLUID RESPONSIVENESS IN SEVERE SEPSIS AND SEPTIC SHOCK PRINCIPAL INVESTIGATOR: JEFFREY C. FRIED, MD DATE OF PROTOCOL: 12/29/2016 VERSION 3 BACKGROUND One of the most challenging problems in critical care is determination of whether patients have adequate intravascular volume, and whether they would benefit from additional intravenous fluids. Giving too much fluid to patients with adequate intravascular volume could lead to fluid overload, congestive heart failure and subsequent respiratory failure. Not giving enough fluid to 1 IRB# 15-21 patients, risks leaving them under-resuscitated and worsening their shock state, leading to organ failures, such as kidney failure. Clinically, it is very difficult to determine this “intravascular volume status”, and predict which patients would benefit from more fluid. One of the ways we currently determine this, is to measure cardiac output and one of its main components, stroke volume (how much blood is pumped by the heart each beat) and see whether these increase in response to a passive leg raise (PLR) maneuver1. The maneuver is simple, quick, with essentially no risk. The maneuver involves measuring baseline cardiac output and stroke volume with the patient’s head up at 45 degrees and legs flat for 3 minutes. Subsequently, the head is placed flat and the legs are raised to 45 degrees for 3 minutes. If the cardiac output and stroke volume increase in response to this maneuver, there is a high probability that the patient will have a good response to giving intravenous fluid. Currently there are multiple FDA approved devices which can be used to measure cardiac output and stroke volume, ranging from very invasive (pulmonary artery catheter), to minimally invasive (requiring arterial catheter +/- central venous catheter- Flotrac, PICCO)3,4 to a newer, completely non- invasive device, called the NICOM (Cheetah). -

Passive Leg Raising for Fluid Responsiveness in Children: Is It Reliable? Javed Ismail1, Arun Bansal2
EDITORIAL Passive Leg Raising for Fluid Responsiveness in Children: Is it Reliable? Javed Ismail1, Arun Bansal2 Indian Journal of Critical Care Medicine (2020): 10.5005/jp-journals-10071-23430 Intravenous fluid administration is an integral part of management for critically ill children with impaired perfusion. Loss of more than 1Pediatric Intensive Care, NMC Royal Hospital, Abu Dhabi, United Arab 15–20% of intravascular volume impairs the preload which in turn Emirates results in decreased cardiac output, clinically manifesting with 2Department of Pediatrics, Advanced Pediatric Centre, Postgraduate signs of impaired perfusion or shock. In children, major causes Institute of Medical Education and Research, Chandigarh, India of shock, such as diarrhea, hemorrhage, sepsis, or anaphylaxis, Corresponding Author: Arun Bansal, Department of Pediatrics, result in either actual or relative intravascular hypovolemia (due to Advanced Pediatric Centre, Postgraduate Institute of Medical vasodilation). Rapid administration of fluid bolus could augment Education and Research, Chandigarh, India, Phone: +91 9815455002, preload to improve the cardiac output and, thus, can potentially e-mail: [email protected] reverse shock. However, studies demonstrate that only half of How to cite this article: Ismail J, Bansal A. Passive Leg Raising for the patients are preload responsive. In the other half, this fluid Fluid Responsiveness in Children: Is it Reliable? Indian J Crit Care Med accumulates and leaks into the interstitial space of almost all organs 2020;24(5):291–292. and can result in serious adverse effects. Fluid leak is exacerbated Source of support: Nil in inflammatory states such as sepsis; and about 95% of the given Conflict of interest: None fluid leaks out within 1 hour even among the fluid responders, thus limiting the beneficial effect.1 Hence, to identify which patient would respond to fluid bolus, tests of fluid responsiveness are recommended. -

Shock and Awe: a Dynamic Approach to Resuscitation
Shock and Awe: A dynamic approach to resuscitation Critical Care Symposium October 28, 2017 Anna Perrello, RPA-C, MPAS Brian Kersten, PharmD, BCCCP, BCPS Disclosures • Brian Kersten o Nothing to disclose • Anna Perrello o Nothing to disclose Objectives • Identify and explain the physiology of various shock states including distributive, cardiogenic, obstructive and hypovolemic. • Discuss advantages and limitations to static and dynamic predictors of volume responsiveness. • Recognize techniques related to visualization of basic structures and medium identification during bedside ultrasonography. • Evaluate treatment options for shock states using dynamic measures for fluid resuscitation Shock • A heterogenous syndrome best defined as circulatory failure o Originates from mismatch between oxygen delivery (DO2) and oxygen consumption (VO2) • Often becomes apparent in setting of arterial hypotension Differentiating Shock Systemic Mixed Wedge Cardiac vascular venous pressure output resistance oxygen Hypovolemic - Hemorrhage ↓ ↓ ↑ ↓ - Dehydration Cardiogenic - Myocardial infarction ↑ ↓ ↑ ↓ - Arrhythmia - Cardiomyopathy Obstructive - Pulmonary embolism ↑↔ ↓ ↑ ↓ - Tension pneumothorax - Cardiac tamponade Distributive - Septic shock - Anaphylaxis ↑↔ ↑ ↓ ↑ - Neurogenic - Myxedema coma - Post-cardiopulmonary bypass Goals of Therapy in Shock • Restore effective tissue perfusion and normalize cellular metabolism by ensuring systemic oxygen delivery by 1. Aggressive and appropriate fluid resuscitation 2. Supporting CO and MAP • Above are titrated to -

Cardiac Output Venous Return
Circulatory Physiology As a Country Doc Episode 3 Cardiac Output and Venous Return Patrick Eggena, M.D. Novateur Medmedia, LLC. Circulatory Physiology As a Country Doc Episode 3 Cardiac Output and Venous Return Patrick Eggena, M.D. Novateur Medmedia, LLC. i Copyright This Episode is derived from: Course in Cardiovascular Physiology by Patrick Eggena, M.D. © Copyright Novateur Medmedia, LLC. April 13, 2012 The United States Copyright Registration Number: PAu3-662-048 Ordering Information via iBooks: ISBN 978-0-9663441-2-7 Circulatory Physiology as a Country Doc, Episode 3: Cardiac Output and Venous Return Contact Information: Novateur Medmedia, LLC 39 Terry Hill Road, Carmel, NY 10512 email: [email protected] Credits: Oil Paintings by Bonnie Eggena, PsD. Music by Alan Goodman from his CD Under the Bed, Cancoll Music, copyright 2005 (with permission). Illustrations, movies, text, and lectures by Patrick Eggena, M.D. Note: Knowledge in the basic and clinical sciences is constantly changing. The reader is advised to carefully consult the instruc- tions and informational material included in the package inserts of each drug or therapeutic agent before administration. The Country Doctor Series illustrates Physiological Principles and is not intended as a guide to Medical Therapeutics. Care has been taken to present correct information in this book, however, the author and publisher are not responsible for errors or omissions or for any consequence from application of the information in this work and make no warranty, expressed or implied, with re- spect to the contents of this publication or that its operation will be uninterrupted and error free on any particular recording de- vice. -

Non-Invasive Stroke Volume Measurement and Passive Leg
Available online http://ccforum.com/content/13/4/R111 ResearchVol 13 No 4 Open Access Non-invasive stroke volume measurement and passive leg raising predict volume responsiveness in medical ICU patients: an observational cohort study Steven W Thiel, Marin H Kollef and Warren Isakow Pulmonary and Critical Care Division, Washington University School of Medicine, Campus Box 8052, 660 South Euclid Avenue, St. Louis, MO 63110, USA Corresponding author: Warren Isakow, [email protected] Received: 19 May 2009 Revisions requested: 22 Jun 2009 Revisions received: 25 Jun 2009 Accepted: 8 Jul 2009 Published: 8 Jul 2009 Critical Care 2009, 13:R111 (doi:10.1186/cc7955) This article is online at: http://ccforum.com/content/13/4/R111 © 2009 Thiel et al.; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Introduction The assessment of volume responsiveness and during PLR was then compared with the change in SV with the decision to administer a fluid bolus is a common dilemma volume expansion to determine the ability of PLR in conjunction facing physicians caring for critically ill patients. Static markers with SV measurement to predict volume responsiveness. of cardiac preload are poor predictors of volume responsiveness, and dynamic markers are often limited by the Results A total of 102 fluid challenges in 89 patients were presence of spontaneous respirations or cardiac arrhythmias. evaluated. In 47 of the 102 fluid challenges (46.1%), SV Passive leg raising (PLR) represents an endogenous volume increased by ≥15% after volume infusion (responders). -

Navigating Preload Assessment
NAVIGATING PRELOAD ASSESSMENT CHOOSING THE RIGHT PATHWAY Cecilia Baylon & Sarah Neville LEARNING OBJECTIVES • Explain the relationship between preload and fluid responsiveness (FR) • Review the different methods of assessing preload and FR • Analyze the current research in regard to their use in the critical care setting O2 demand CARDIAC OUTPUT Respiratory Lung Muscle Compliance Patient’s Venous Vessel Diameter Function Pre-existing Alveoli Return (Peripheral Medical Perfused? Vascular Condition Blood Work of Resistance) Viscosity Breathing Total Alveoli Thickness of Circulating Ventilated? Alveolar-Capillary Membrane Volume Aortic Physical Tidal Volume etc Impedence Activity Respiratory Vital Capacity Rate Functional Resid. Capac V/Q Emotional Diffusion pH, PC02 Matching Contractility Preload Temperature Stress 2,3 DPG Afterload Ventilation ie: pain PaCO2 Oxygen ALVEOLAR Hgb Hgb Level GAS EXCHANGE Affinity METABOLIC STROKE HEART RATE DEMANDS Pa02 Oxygen VOLUME ARTERIAL OXYGEN transported SATURATION in blood Sa02 ARTERIAL OXYGEN CARDIAC OUTPUT CONTENT OXYGEN OXYGEN SUPPLY DEMAND BALANCE End organ perfusion OXYGEN SUPPLY & DEMAND (HEMODYNAMIC) FRAMEWORK CONTRACTILITY PRELOAD AFTERLOAD STROKE VOLUME X HEART RATE CARDIAC OUTPUT EOP FRANK-STARLING’S LAW FRANK-STARLING’S LAW • “the force of ventricular ejection is directly related to…” VOLUME IN THE VENTRICLE AT END-DIASTOLE (PRELOAD) AMOUNT OF MYOCARDIAL STRETCH PLACED ON THE VENTRICLE AS A RESULT Urden, Stacy, Lough (2018), p. 214 Ejection Phase Hyperinotropy Contractility Norminotropy