Pharmavoice 100 Honorees, Including Our Own Kara Dennis and Steve Smith!
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2009: Turning the Corner
data page 2009: Turning the corner Walter Yang Despite the shaky start to 2009, the biotech sector regained its financial Although initial public offerings showed signs of resuscitation (at least footing. Biotech indices were up, as were offerings and partnership mon- 13 more companies are now in the queue), follow-on financings came in ies. Excluding collaborations, the sector raised a total of $24.3 billion. above $6 billion—the second-best year over the past decade. Stock market performance Global biotech industry financing Although biotech indices were up ~16% last year, they underperformed The boost in partnership promises to US biotechs and follow-on financings other major indices. pushed industry funding to $61.3 billion, up 82% from 2008. 1,500 Swiss Market S&P 500 2009 36.9 10.0 5.1 2.2 6.0 0.9 1,400 NASDAQ Biotech Dow Jones 2008 20.0 3.2 5.3 3.1 1.9 0.1 1,300 NASDAQ BioCentury 100 2007 22.4 11.7 6.8 4.7 4.4 3.0 1,200 Partnering 2006 19.8 11.9 5.6 4.7 5.6 2.0 1,100 Year Debt and other Index 17.3 6.1 5.4 2.7 4.8 1.9 1,000 2005 Venture capital PIPEs 900 2004 10.9 8.8 5.3 2.9 3.3 2.6 Follow-ons 800 2003 8.9 9.1 4.0 2.2 3.9 0.5 IPOs 700 010203040506070 Amount raised ($ billions) 1/09 2/09 3/09 4/09 5/09 6/09 7/09 8/09 9/09 12/08 Month ending 10/09 11/09 12/09 Partnership figures are for deals involving a US company. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

Schedule 14A
QuickLinks -- Click here to rapidly navigate through this document UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 SCHEDULE 14A Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934 (Amendment No. ) Filed by the Registrant ý Filed by a Party other than the Registrant o Check the appropriate box: o Preliminary Proxy Statement o Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) ý Definitive Proxy Statement o Definitive Additional Materials o Soliciting Material under §240.14a-12 Verastem, Inc. (Name of Registrant as Specified In Its Charter) (Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box): ý No fee required. o Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: o Fee paid previously with preliminary materials. o Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. -

1 United States District Court for the Southern District
Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 1 of 167 UNITED STATES DISTRICT COURT FOR THE SOUTHERN DISTRICT OF IOWA, CENTRAL DIVISION THE STATE OF IOWA, Plaintiff, v. ABBOTT LABORATORIES, INC., AGOURON PHARMACEUTICALS, INC., JURY TRIAL REQUESTED ALPHARMA, INC., ALZA CORPORATION, AMGEN, INC., ASTRAZENECA L.P., ASTRAZENECA PHARMACEUTICALS, LP., AVENTIS BEHRING L.L.C., COMPLAINT BARR LABORATORIES, INC., BAXTER INTERNATIONAL, INC., BAXTER HEALTHCARE CORPORATION, BAYER CORPORATION, BAYER PHARMACEUTICALS CORPORATION, BEN VENUE LABORATORIES, INC., BOEHRINGER INGELHEIM CORPORATION, BOEHRINGER INGELHEIM PHARMACEUTICALS, INC., BRISTOL-MYERS SQUIBB COMPANY, CENTOCOR, INC., CHIRON CORPORATION, DERMIK LABORATORIES, INC., DEY, INC., DEY, L.P., ELI LILLY AND COMPANY, EMD, INC., ENDO PHARMACEUTICALS, INC., ETHEX CORPORATION, ETHICON, INC., FOREST LABORATORIES, INC., FOREST PHARMACEUTICALS, INC. GENEVA PHARMACEUTICALS, GLAXOSMITHKLINE, PLC, GLAXOWELLCOME, INC., GREENSTONE, LTD., HOECHEST MARION ROIUSSEL, INC., HOFFMAN-LAROCHE, INC., 1 Case 1:07-cv-12141-PBS Document 18-3 Filed 11/28/07 Page 2 of 167 IMMUNEX CORPORATION, IVAX CORPORATION, IVAX PHARMACEUTICALS, INC., JANSSEN PHARMACEUTICA PRODUCTS, LP, JOHNSON & JOHNSON, KING PHARMACEUTICALS, INC., KING RESEARCH AND DEVELOPMENT, MCNEIL-PPC, INC., MEDIMMUNE, INC., MERCK & CO., INC., MONARCH PHARMACEUTICALS, INC., MYLAN LABORATORIES, INC., MYLAN PHARMACEUTICALS, INC., NOVARTIS PHARMACEUTICALS CORPORATION, NOVOPHARM USA, INC., ONCOLOGY THERAPEUTICS NETWORK CORP., ORTHO-MCNEIL PHARMACEUTICAL, INC., -

28 May 2009 Thomas E. Costa
The Third International Pharmaceutical Regulatory and Compliance Congress and Best Practices Forum Thomas E. Costa 2828 MayMay 20092009 This presentation represents my own personal opinion and is not the official position of Bristol-Myers Squibb PhRMA Code 2009 • Updated in response to concerns of healthcare stakeholders • Reaffirms that interactions between pharmaceutical company representatives and healthcare professionals (HCPs) should: ¾ Inform HCPs about the benefits and risks of our products ¾ Provide scientific and educational information ¾ Obtain feedback and advice about our products through consultation with medical experts • The PhRMA Code is the new industry standard PhRMA Code Signatory Companies • Abbott • Eli Lilly and Company • Amgen, Inc. • Merck & Company, Inc. • Amylin Pharmaceuticals, Inc. • Millennium Pharmaceuticals, Inc. • Astellas US LLC • Novartis Pharmaceuticals • AstraZeneca LP Corporation • Bayer HealthCare • Novo Nordisk Inc. Pharmaceuticals • Otsuka America, Inc. • Boehringer Ingleheim • Ovation Pharmaceuticals, Inc. Pharmaceuticals, Inc. • Pfizer, Inc. • Bristol-Myers Squibb Company • Purdue Pharma LP • Cephalon, Inc. • sanofi-aventis US • Covidien Ltd. • Schering-Plough Corporation • Daiichi Sankyo, Inc. • Sepracor, Inc. • Eisai, Inc. • Signa-Tau Pharmaceuticals, Inc. • EMD Serono • Solstice Neurosciences, Inc. • Endo Pharmaceuticals, Inc. • Solvay Pharmaceuticals, Inc. • Genzyme Corporation • Takeda Pharmaceuticals North • GlaxoSmithKline America, Inc. • Hoffmann-La Roche, Inc. • Wyeth • Johnson and Johnson -

Update Covers C1-C4 Iss2 2005.Indd
In Compliance and In Court by John B. Reiss, Ph.D., and William M. Janssen his article examines various fraud and abuse cases is the simultane- violated the Anti-Kickback Statute. Tlegal issues facing the medical ous fi ling of False Claims Act charges. Under the government’s theory, these device, pharmaceutical, and biologics Because the False Claims Act permits inducements resulted in an illegal use industries; court decisions or settle- fi nes of up to three times the amount of the drugs, and claims fi led for these ments over the past year; and suggested of the claim—and penalties of between uses were thus false. Also brought actions. New guidelines set standards $5,500 and $11,000 per claim—penal- into focus by this case were the False that industry leaders should consider as ties can add up quickly to billions of Claims Act’s qui tam provisions, which they plan product development, sales, dollars, giving the government an enor- allow whistleblowers to participate in and marketing strategies. mous “hammer” with which to exact up to 25% of the government’s recov- settlements. ery. A majority of the recent cases have Fraud and Abuse and the The complexity of the application resulted from whistleblower fi lings. False Claims Act of these laws is demonstrated by the TAP Pharmaceuticals made an Fraud and abuse issues dominated Warner-Lambert case.1 The primary $875 million payment in October 2001, the headlines during 2004. The U.S. allegation involved the company’s partly as the result of its guilty plea for Attorney and the Department of Health off-label marketing of its epilepsy violating the Prescription Drug Market- and Human Services (DHHS) Offi ce of drug, Neurontin®, in violation of the ing Act.2 The government also alleged Inspector General (OIG) continued a Federal Food, Drug, and Cosmetic Act manipulation of the average wholesale high level of activity, and states’ attor- (FDCA). -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -
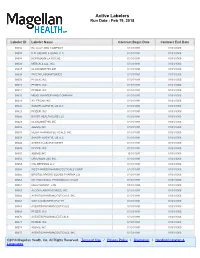
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Bioscience Industry Companies This List Includes Georgia Companies That Fall Into the Bioscience Industry, with a Brief Description Shown
Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Company Name City Description Website Develops novel automated technology for the 3 Ti (Transfusion Transplantation Technologies) Atlanta diagnostic testing of blood associated with blood http://www.3tibio.com/location.html transfusions. Develops 3-D modeling technology for dental 360 Imaging Atlanta http://www.360imaging.com surgeries. Develops real-time 3-D visualization technology for 3D SURGICAL SOLUTIONS Marietta minimally invasive surgeries. Develops and manufactures skin-safe tape for 3M Atlanta healthcare. http://www.3m.com 3R Gloves Roswell Manufactures reusable and recyclable latex gloves. 4P Therapeutics Norcross Manufacturer of drug delivery technologies www.4ptherapeutics.com Manufactures blood pressure monitors, scales, A&D Engineering Duluth www.andonline.com thermometers, and activity monitors. Manufactures and specializes in the production of A&L Shielding Rome www.alshielding.com radiation shielding products. Develops and produces diamond and carbide A&M INSTRUMENTS Alpharetta www.aminstr.com cutting tools. Manufactures hospital air purification equipment Abatement Technologies Suwanee www.abatement.com for infection control. Science based offerings in diagnostics, medical Abbott Laboratories Alpharetta www.abbott.com devices, nutrition, and pharmaceuticals. Bioscience Industry Companies This list includes Georgia companies that fall into the bioscience industry, with a brief description shown. Develops proprietary technology to discover Abeome Athens www.abeomecorp.com therapeutic and diagnostic monoclonal antibodies. Manufactures medical devices and provides Accellent Trenton research for several non-medical industries. Savannah and Augusta Testing services for pharmaceutical and Acuren Inspection www.acuren.com Metro biotechnology companies. Pharmaceutical research focused on cell Aderans Pharmaceutical Research Institute Marietta engineering solutions for hair loss. -

Why Are Some Generic Drugs Skyrocketing in Price? Hearing Committee on Health, Education, Labor, and Pensions United States Sena
S. HRG. 113–859 WHY ARE SOME GENERIC DRUGS SKYROCKETING IN PRICE? HEARING BEFORE THE SUBCOMMITTEE ON PRIMARY HEALTH AND AGING OF THE COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS UNITED STATES SENATE ONE HUNDRED THIRTEENTH CONGRESS SECOND SESSION ON EXAMINING THE PRICING OF GENERIC DRUGS NOVEMBER 20, 2014 Printed for the use of the Committee on Health, Education, Labor, and Pensions ( Available via the World Wide Web: http://www.gpo.gov/fdsys/ U.S. GOVERNMENT PUBLISHING OFFICE 24–459 PDF WASHINGTON : 2017 For sale by the Superintendent of Documents, U.S. Government Publishing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Nov 24 2008 13:32 May 19, 2017 Jkt 000000 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 S:\DOCS\24459.TXT DENISE HELPN-003 with DISTILLER COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS TOM HARKIN, Iowa, Chairman BARBARA A. MIKULSKI, Maryland LAMAR ALEXANDER, Tennessee PATTY MURRAY, Washington MICHAEL B. ENZI, Wyoming BERNARD SANDERS (I), Vermont RICHARD BURR, North Carolina ROBERT P. CASEY, JR., Pennsylvania JOHNNY ISAKSON, Georgia KAY R. HAGAN, North Carolina RAND PAUL, Kentucky AL FRANKEN, Minnesota ORRIN G. HATCH, Utah MICHAEL F. BENNET, Colorado PAT ROBERTS, Kansas SHELDON WHITEHOUSE, Rhode Island LISA MURKOWSKI, Alaska TAMMY BALDWIN, Wisconsin MARK KIRK, Illinois CHRISTOPHER S. MURPHY, Connecticut TIM SCOTT, South Carolina ELIZABETH WARREN, Massachusetts DEREK MILLER, Staff Director LAUREN MCFERRAN, Deputy Staff Director and Chief Counsel DAVID P. CLEARY, Republican Staff Director SUBCOMMITTEE ON PRIMARY HEALTH AND AGING BERNARD SANDERS, Vermont, Chairman BARBARA A. -

Beyond Borders Global Biotechnology Report 2010
Beyond borders Global biotechnology report 2010 To our clients and friends As economies around the world begin to recover from the worst diminished means, key constituents in the biotechnology economic crisis since the Great Depression, it is clear that a “new ecosystem — investors, pharmaceutical companies, payors, normal” is emerging. Among other things, this is a world in which governments and biotech firms — need to do more with less. capital flows are far more constrained than during the easy money- Companies that are able to develop creative solutions to boost fueled decade that preceded the downturn. What impact has this efficiency will be best positioned to succeed in the new normal. capital-constrained environment had on biotechnology — a business Readers of Beyond borders will notice some structural changes that is based on an enormous hunger for capital coupled with an in this year’s report. In prior years, we organized the report by exceptionally long path to commercial payback? geography, with sections on the Americas, Europe and Asia-Pacific. We set out to answer that question in this year’s Beyond borders. This year, we have instead organized the report thematically. As usual, we analyze key indicators of the industry’s performance: The Global perspective section brings together our point of view financing activity, deal trends, financial results, pipeline strength on salient trends and implications as well as some perspectives and product approvals. While the overall results are robust, the gap from company leaders. This is followed by a Country profiles between the industry’s haves and its have-nots has widened. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC.