Climate-Driven Statistical Models As Effective Predictors of Local Dengue Incidence in Costa Rica: a Generalized Additive Model and Random Forest Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mapa De Valores De Terrenos Por Zonas Homogéneas Provincia 3 Cartago Cantón 03 La Unión
MAPA DE VALORES DE TERRENOS POR ZONAS HOMOGÉNEAS PROVINCIA 3 CARTAGO CANTÓN 03 LA UNIÓN 496200 501200 Mapa de Valores de Terrenos Goicoechea por Zonas Homogéneas Provincia 3 Cartago Goicoechea Cantón 03 La Unión Ministerio de Hacienda Órgano de Normalización Técnica Montes de Oca QUEBRADA CHISPAFINCA LOS LOTES CALLE EL CERRITO URB. APASIBLES VALLES URB. SOL DEL ESTE 3 03 07 U04 CALLE LIMBURGIA CALLE DIAZ CALLE LOS JAULES 3 03 07 R05/U05 URB. MANSIONES n CALLE PIZOTE 3 03 06 R03 URB. LAS CUMBRES QUEBRADA CHISPA A SABANILLA æ CALLE LA MINITA RESID. VALLE ESCONDIDO 3 03 07 U02 3 03 07 U03 PLAZA DE FÚTBOL DE SAN RAMÓN CALLE LA FE 3 03 07 U01 3 03 07 U11 3 03 07 U12 CENTRO PASTORAL EL REDIL 3 03 07 U06 RESID. EL REFUGIO æ URB. LAS CUMBRES URB.VILLA HERMOSA CALLE SAN MIGUEL CALLE EL FRESAL COND. LAS RUSIAS COLEGIO MARIAM BAKER SCHOOL 3 03 07 U10 n URB. LAS CUMBRES QUEBRADA VEGA URB. VERDE VISTA 3 03 07 U07 HOSPITAL DR. ROBERTO CHACON PAUT 3 03 07 U09 $1 CALLE LAS RUSIAS CALLE LOS POLICIAS (LOS NARANJOS) CALLE ALVARADO QUEBRADA GRANADILLA COND. LAKOTA QUEBRADA VEGA CALLE VIQUEZ CALLE NARANJO LA CIMA 2 HOGAR CREA 3 03 05 U04 $1 CALLE EL BOSQUE COND. BARLOVENTO 1098400 3 03 05 U11 3 03 05 U05 1098400 Aprobado por: 3 03 07 U08 3 03 05 R03/U03 URB. LOS ALAMITOS CALLE NARANJO IEM n 3 03 05 U06 QUEBRADA MUNECO Ing. Alberto Poveda Alvarado 3 03 05 U10 A SAN JOSÉ 3 03 05 U17 COLEGIO ALEJANDRO QUESADA 3 03 06 R02 Director n RIO MARIA AGUILAR LICEO FRANCO COSTARRICENSE 3 03 05 U12 CALLE SANCHEZ n BN Órgano Normalización Técnica ; B. -

Plan Cantonal 2013
1 PLAN CANTONAL DE DESARROLLO HUMANO LOCAL DEL CANTÓN DE BELÉN 2013-2022 Realizado con la participación de: Equipo de Gestión Local del Plan Cantonal de Desarrollo Humano de Belén Municipalidad de Belén PNUD y MIDEPLAN Agosto, 2012 Plan Cantonal de Desarrollo Humano Local del Cantón de Belén 2013-2022 2 Índice de contenido PRESENTACIÓN...................................................................................................................3 CAPÍTULO I...........................................................................................................................5 INTRODUCCIÓN..................................................................................................................5 1.1 Metodología:.....................................................................................................................6 1.2 Equipo de Gestión Local de Belén– EGL: .......................................................................7 1.3 Antecedentes de la Planificación en el cantón de Belén...................................................8 1.4. Antecedentes de la formulación del PCDHL de Belén....................................................9 CAPÍTULO II........................................................................................................................11 CONTEXTO NACIONAL Y CANTONAL DEL PLAN DE DESARROLLO HUMANO LOCAL..................................................................................................................................11 2.1 Introducción...................................................................................................................12 -

Nombre Del Comercio Provincia Distrito Dirección Horario
Nombre del Provincia Distrito Dirección Horario comercio Almacén Agrícola Alajuela Aguas Claras Alajuela, Upala Aguas Claras, Cruce Del L-S 7:00am a 6:00 pm Aguas Claras Higuerón Camino A Rio Negro Comercial El Globo Alajuela Aguas Claras Alajuela, Upala Aguas Claras, contiguo L - S de 8:00 a.m. a 8:00 al Banco Nacional p.m. Librería Fox Alajuela Aguas Claras Alajuela, Upala Aguas Claras, frente al L - D de 7:00 a.m. a 8:00 Liceo Aguas Claras p.m. Librería Valverde Alajuela Aguas Claras Alajuela, Upala, Aguas Claras, 500 norte L-D de 7:00 am-8:30 pm de la Escuela Porfirio Ruiz Navarro Minisúper Asecabri Alajuela Aguas Claras Alajuela, Upala Aguas Claras, Las Brisas L - S de 7:00 a.m. a 6:00 400mts este del templo católico p.m. Minisúper Los Alajuela Aguas Claras Alajuela, Upala, Aguas Claras, Cuatro L-D de 6 am-8 pm Amigos Bocas diagonal a la Escuela Puro Verde Alajuela Aguas Claras Alajuela, Upala Aguas Claras, Porvenir L - D de 7:00 a.m. a 8:00 Supermercado 100mts sur del liceo rural El Porvenir p.m. (Upala) Súper Coco Alajuela Aguas Claras Alajuela, Upala, Aguas Claras, 300 mts L - S de 7:00 a.m. a 7:00 norte del Bar Atlántico p.m. MINISUPER RIO Alajuela AGUAS ALAJUELA, UPALA , AGUAS CLARAS, L-S DE 7:00AM A 5:00 PM NIÑO CLARAS CUATRO BOCAS 200M ESTE EL LICEO Abastecedor El Alajuela Aguas Zarcas Alajuela, Aguas Zarcas, 25mts norte del L - D de 8:00 a.m. -

“Marimba Por Tí Me Muero”: Region and Nation in Costa Rica, 1824-1939
“MARIMBA POR TÍ ME MUERO”: REGION AND NATION IN COSTA RICA, 1824-1939 by Soili Iiris Buska Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements for the degree Doctor of Philosophy in the Department of History Indiana University March 2006 Accepted by the Graduate Faculty, Indiana University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ______________________________________ Jeffrey L. Gould, Ph.D., Chairperson ______________________________________ Peter Guardino, Ph.D. Doctoral Committee _______________________________________ Daniel James, Ph.D. ______________________________________ Víctor Hugo Acuña Ortega, Ph.D. December 16, 2005 ii © (2005) Soili Iiris Buska ALL RIGHTS RESERVED iii Acknowledgements Many people and institutions made this dissertation possible. The Department of History of Indiana University provided me with financial support and graduate appointments from the start of my studies in Bloomington. The pre-dissertation grant and research fellowship from the Office of International Programs and the University Graduate School of Indiana University respectively helped me to define my project and to begin the research. The Indiana University Bloomington Library staff and the History Department Staff in IU Bloomington were of greatest help. History Department Graduate Secretary Alexia Bock assisted in many ways and gave me crucial practical advice during my stay in Bloomington. The Center for Latin American Studies of the University of Pittsburgh's University Center for International Studies generously opened its doors to me in the summer of 2005. The Escuela de Historia, Centro de Investigaciones Históricas de America Central (CIHAC), and the Oficina de Asuntos Internacionales y Cooperación Externa (OAICE) of the Universidad de Costa Rica have all participated in making possible research and writing of this dissertation. -
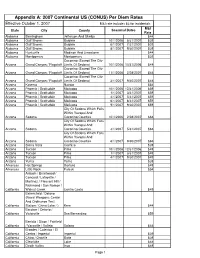
1 Ccr 101-1 State Fiscal Rules 2 (Pdf)
Appendix A: 2007 Continental US (CONUS) Per Diem Rates Effective October 1, 2007 M&I rate includes $3 for incidentals M&I State City County Seasonal Dates Rate Alabama Birmingham Jefferson And Shelby $44 Alabama Gulf Shores Baldwin 10/1/2006 5/31/2007 $39 Alabama Gulf Shores Baldwin 6/1/2007 7/31/2007 $39 Alabama Gulf Shores Baldwin 8/1/2007 9/30/2007 $39 Alabama Huntsville Madison And Limestone $44 Alabama Montgomery Montgomery $39 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 10/1/2006 10/31/2006 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 11/1/2006 2/28/2007 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 3/1/2007 9/30/2007 $44 Arizona Kayenta Navajo $54 Arizona Phoenix / Scottsdale Maricopa 10/1/2006 12/31/2006 $59 Arizona Phoenix / Scottsdale Maricopa 1/1/2007 3/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 4/1/2007 5/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 6/1/2007 8/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 9/1/2007 9/30/2007 $59 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 10/1/2006 2/28/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 3/1/2007 5/31/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 6/1/2007 9/30/2007 $64 Arizona Sierra Vista Cochise $39 Arizona Tucson Pima 10/1/2006 12/31/2006 $49 Arizona Tucson Pima 1/1/2007 3/31/2007 $49 Arizona Tucson Pima 4/1/2007 9/30/2007 $49 Arizona Yuma Yuma -

Redalyc.LOS CAMINOS DE LA HISTORIA LOCAL EN SAN RAMÓN
Diálogos Revista Electrónica de Historia E-ISSN: 1409-469X [email protected] Universidad de Costa Rica Costa Rica Castro Sánchez, Silvia LOS CAMINOS DE LA HISTORIA LOCAL EN SAN RAMÓN Diálogos Revista Electrónica de Historia, octubre, 2014, pp. 3-18 Universidad de Costa Rica San Pedro de Montes de Oca, Costa Rica Disponible en: http://www.redalyc.org/articulo.oa?id=43932584001 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto LOS CAMIOS DE LA HISTORIA LOCAL E SA RAM ROADS O THE LOCAL HISTORY I SA RAM Silvia Castro Sánchez Palabras clave Historia local, metodología histórica, historia local académica, historia local no académica. eyords Local history, historical methods, academic local history, nonacademic local history. eca de recepción: 7 de febrero, 2014 - eca de aceptación: 1 de junio, 2014 Resumen Este artículo presenta un repaso de las formas en que se ha escrito la historia del cantón de San Ramón, desde 1917 al 2009.Mediante una cronología dividida en tres períodos, se discuten los estilos empleados en las historias de este lugar. Se destacan, especialmente, las formas en que trabajan historiadores locales académicos, así como los historiadores locales “por vocación” o sin formación académica. También se incluyen algunas reexiones para fortalecer el estudio de la historia local de San Ramón, ya que esta, pese a su trayectoria, proporciona un conocimiento fragmentario del pasado de esta comunidad. -

Pdf Laboratories Authorized in Costa Rica for Test SARS-COV-2
Updated September 17, 2021 Legend: White: labs authorized to perform PCR testing Green: labs authorized to perform antigen testing only Orange: labs authorized to perform both tests (PCR & antigen) Caribbean Authorized Lab name (Sample Address Telephone WhatsApp Social Media Online map partner lab taking center) Laboratorios Laboratorio Dr. Eric Dirección: 150 m al norte del correo de 2758-3106 - Servicios Médicos Ver ubicación Echandi Castro Limón Limonenses | Facebook Laboratorios Laboratorio Bioclinic 75 metros sur de la Danny Hayling, 2550-0450 8893-1800 Laboratorio Bioclinic Ver ubicación Echandi Clínica Santa Teresa, Limón Centro. 8423-3861 Puerto Viejo | Facebook Laboratorios Laboratorio Bioclinic 500 metros sur de la casa de la cultura 2750-0450 8893-1800 Laboratorio Bioclinic Echandi Puerto Viejo Centro Comercial Puerto Viejo Shops 8423-3861 Puerto Viejo | Ver ubicación Facebook South Pacific Authorized Lab name (Sample Address Telephone WhatsApp Social Media Online map partner lab taking center) Laboratorios Laboratorio Calderón Diagonal al ministerio de salud, San 2771-2030 6306-0781 Laboratorio Clínico Echandi González Isidro de Pérez Zeledón. Calderón González #2 | Ver ubicación Facebook Laboratorios Laboratorio Clínico Detrás de la Musoc, frente a Toys, San 2772-0909 8912-0909 Laboratorio Biotest | Ver ubicación Echandi Biotest Isidro del General Perez Zeledón Facebook Busque este sello para identificar a los laboratorios aprobados. Looks for this seal to identify approved laboratories. Laboratorios EXPRESSLAB 50 metros al sur del estadio municipal de 2771-0120 8967-2525 ExpressLab | Facebook Ver ubicación San José Pérez Zeledón Central Pacific Authorized Lab name (Sample Address Telephone WhatsAp Social Media Online map partner lab taking center) p Hospital Hospital Metropolitano, Quepos, Puntarenas. -

Provincia Nombre Provincia Cantón Nombre Cantón Distrito Nombre
Provincia Nombre Provincia Cantón Nombre Cantón Distrito Nombre Distrito Barrio Nombre Barrio 1 San José 1 San José 1 CARMEN 1 Amón 1 San José 1 San José 1 CARMEN 2 Aranjuez 1 San José 1 San José 1 CARMEN 3 California (parte) 1 San José 1 San José 1 CARMEN 4 Carmen 1 San José 1 San José 1 CARMEN 5 Empalme 1 San José 1 San José 1 CARMEN 6 Escalante 1 San José 1 San José 1 CARMEN 7 Otoya. 1 San José 1 San José 2 MERCED 1 Bajos de la Unión 1 San José 1 San José 2 MERCED 2 Claret 1 San José 1 San José 2 MERCED 3 Cocacola 1 San José 1 San José 2 MERCED 4 Iglesias Flores 1 San José 1 San José 2 MERCED 5 Mantica 1 San José 1 San José 2 MERCED 6 México 1 San José 1 San José 2 MERCED 7 Paso de la Vaca 1 San José 1 San José 2 MERCED 8 Pitahaya. 1 San José 1 San José 3 HOSPITAL 1 Almendares 1 San José 1 San José 3 HOSPITAL 2 Ángeles 1 San José 1 San José 3 HOSPITAL 3 Bolívar 1 San José 1 San José 3 HOSPITAL 4 Carit 1 San José 1 San José 3 HOSPITAL 5 Colón (parte) 1 San José 1 San José 3 HOSPITAL 6 Corazón de Jesús 1 San José 1 San José 3 HOSPITAL 7 Cristo Rey 1 San José 1 San José 3 HOSPITAL 8 Cuba 1 San José 1 San José 3 HOSPITAL 9 Dolorosa (parte) 1 San José 1 San José 3 HOSPITAL 10 Merced 1 San José 1 San José 3 HOSPITAL 11 Pacífico (parte) 1 San José 1 San José 3 HOSPITAL 12 Pinos 1 San José 1 San José 3 HOSPITAL 13 Salubridad 1 San José 1 San José 3 HOSPITAL 14 San Bosco 1 San José 1 San José 3 HOSPITAL 15 San Francisco 1 San José 1 San José 3 HOSPITAL 16 Santa Lucía 1 San José 1 San José 3 HOSPITAL 17 Silos. -

Informe Final
Estudio de valoración para la creación de un Centro Académico del Instituto Tecnológico de Costa Rica en la Región Chorotega Informe Final Estudio de valoración para la creación de un Centro Académico del Instituto Tecnológico de Costa Rica en la Región Chorotega Informe Final Febrero, 2019 Tecnológico de Costa Rica Apto. 159-7050 Cartago, Costa Rica www.tec.ac.cr Estudio de valoración para la creación de un Centro Académico 4 del Instituto Tecnológico de Costa Rica en la Región Chorotega Fotos de portada Casona de Santa Rosa, José A. Sánchez (2017); Iglesia Colonial de San Blas de Nicoya, Alfredo Villarreal (2014); Eólicas Tilarán, Agencia GR (2018); Árbol de Guanacaste, Freddy Rojas (2011). 658.159 S211es Sánchez Sanabria, José Antonio Estudio de valoración para la creación de un Centro Académico del Instituto Tecnológico de Costa Rica en la Región Chorotega / José Antonio Sánchez Sanabria. -- 1 edición -- Cartago, Costa Rica: Editorial Tecnológica de Costa Rica, 2019 92 páginas: ilustraciones, diagramas, tablas, gráficos. Referencias bibliográficas Anexos ISBN: 978-9930-541-28-9 ISBN: 978-9930-541-29-6 (ebook) 1. Administración Universitaria 2. Costa Rica-Región Chorotega 3. Estudios regionales 4. Variables de estudio I. Título Tecnológico de Costa Rica 5 Contenidos| Contenidos .................................................................................................................................................5 Presentación ............................................................................................................................................11 -

Codigos Geograficos
División del Territorio de Costa Rica Por: Provincia, Cantón y Distrito Según: Código 2007 Código Provincia, Cantón y Distrito COSTA RICA 1 PROVINCIA SAN JOSE 101 CANTON SAN JOSE 10101 Carmen 10102 Merced 10103 Hospital 10104 Catedral 10105 Zapote 10106 San Francisco de Dos Ríos 10107 Uruca 10108 Mata Redonda 10109 Pavas 10110 Hatillo 10111 San Sebastián 102 CANTON ESCAZU 10201 Escazú 10202 San Antonio 10203 San Rafael 103 CANTON DESAMPARADOS 10301 Desamparados 10302 San Miguel 10303 San Juan de Dios 10304 San Rafael Arriba 10305 San Antonio 10306 Frailes 10307 Patarrá 10308 San Cristóbal 10309 Rosario 10310 Damas 10311 San Rafael Abajo 10312 Gravilias 10313 Los Guido 104 CANTON PURISCAL 10401 Santiago 10402 Mercedes Sur 10403 Barbacoas 10404 Grifo Alto 10405 San Rafael 10406 Candelaria 10407 Desamparaditos 10408 San Antonio 10409 Chires 105 CANTON TARRAZU 10501 San Marcos 10502 San Lorenzo 10503 San Carlos 106 CANTON ASERRI 10601 Aserrí 10602 Tarbaca o Praga 10603 Vuelta de Jorco 10604 San Gabriel 10605 La Legua 10606 Monterrey 10607 Salitrillos 107 CANTON MORA 10701 Colón 10702 Guayabo 10703 Tabarcia 10704 Piedras Negras 10705 Picagres 108 CANTON GOICOECHEA 10801 Guadalupe 10802 San Francisco 10803 Calle Blancos 10804 Mata de Plátano 10805 Ipís 10806 Rancho Redondo 10807 Purral 109 CANTON SANTA ANA 10901 Santa Ana 10902 Salitral 10903 Pozos o Concepción 10904 Uruca o San Joaquín 10905 Piedades 10906 Brasil 110 CANTON ALAJUELITA 11001 Alajuelita 11002 San Josecito 11003 San Antonio 11004 Concepción 11005 San Felipe 111 CANTON CORONADO -

Mapa Del Cantón Tibás 13, Distrito 01 a 05
MAPA DE VALORES DE TERRENOS POR ZONAS HOMOGÉNEAS PROVINCIA 1 SAN JOSÉ CANTÓN 13 TIBÁS 488000 489000 490000 491000 492000 493000 494000 MINISTERIO DE HACIENDA DIRECCIÓN GENERAL DE TRIBUTACIÓN SAN PABLO DIVISIÓN ÓRGANO DE NORMALIZACIÓN TÉCNICA 1103000 1103000 TIBÁS o d a n o r o C BENEFICIO LIBERTAD A Silo Ing. Alberto Poveda Alvarado DIRECTOR ONT rilla SANTO DOMINGO Vi Río n ó m i L A A S an Tajo to Ing. Wanny Chaves Solano D om in SUBDIRECTORA go Relleno Sanitario 113-01-U08113-01-U08 VALORACIONES MUNICIPALES ALAMEDAS Talleres VIRGINIA 113-01-U19113-01-U19 113-01-U07113-01-U07 LINDAVISTA FLORIDA Río V a Parque ir d i e lla Escuela l o Jesús b r C 113-01-U04 A Jiménez 113-01-U04 a Ing. Luis Coto Muñoz e k l l æ l l e Villa Franca a C Parque C asito o COORDINADOR GRUPO le Tib Imprenta Lemon n Orfanato l g 1102000 1102000 Ca Condominio Real de Colima a Estadio Ricardo Saprissa DE PLATAFORMA DE VALORES Plaza PARQUES DEL NORTE da 7 Escuela Estadio veni k A 113-01-U05113-01-U05 113-01-U06113-01-U06 VILLA FRANCA 113-01-U20113-01-U20 113-01-U21113-01-U21 Luces y LA ARBOLEDA 113-01-U09113-01-U09 Escuela Sistemas de 113-01-U03 Monte ESCALA 1:7.000 Plaza LINDAVISTA 113-01-U03 æ Costa Rica Urbanización María Lilly LAS ACACIAS Verdek SAN JUAN a INVU LA FLORIDA Avenida 5 n Metros e Clínica Tibás r Los Andes A 7035 0 70 140 210 Escuela e Panadería Parque l k SAN JERÓNIMO l a C Kinder Asilo Escuela 1 cm en el mapa equivale a 70 m en terreno Avenida 3 Urbanización Piolín José R. -

Regiones Y Cantones De Costa Rica
DIRECCIÓN DE GESTIÓN MUNICIPAL SECCIÓN DE INVESTIGACIÓN Y DESARROLLO REGIONES Y CANTONES DE COSTA RICA SERIE CANTONES DE COSTA RICA : N° 2 2003 DIRECCIÓN DE GESTIÓN MUNICIPAL SECCIÓN DE INVESTIGACIÓN Y DESARROLLO REGIONES Y CANTONES DE COSTA RICA Elaborado por: Ronulfo Alvarado Salas SERIE CANTONES DE COSTA RICA : N° 2 2003 CONTENIDO Página N° PRESENTACIÓN 1 1 El Proceso de Regionalización en Costa Rica 3 2 Sobre el concepto de región 7 3 Información básica sobre las regiones en Costa Rica 10 ANEXOS N°1 Mapa sobre las regiones en Costa Rica 51 N°2 Población y territorio según región 52 N°3 Población y territorio según provincia 53 BIBLIOGRAFÍA 53 PRESENTACIÓN En el primer documento de esta serie sobre cantones de Costa Rica, se analizaron algunos de los inconvenientes que presenta la actual división territorial administrativa para efectos, entre otros cosas, de planificar y coordinar los programas de desarrollo que llevan a cabo las diversas instituciones del país. Es corriente que sobre un mismo territorio se proyecten diversas instituciones con grados diferentes de competencias y especialización, sin que hasta el momento se haya podido obtener una acción armónica y eficaz entre ellas. En algunas provincias hasta 44 instituciones públicas (ministerios, entidades descentralizadas, municipalidades) realizan sus labores sin una coordinación estratégica y operativa que garantice el mejor uso de los cuantiosos recursos que están implicados. La regionalización se planteó como una forma de mejorar los procesos de planificación y coordinación interinstitucional, no solo para garantizar un mejor uso de los recursos públicos sino también para procurar un desarrollo más equitativo y armonioso entre las diferentes regiones del país, evitando en lo posible las brechas o desequilibrios regionales que una serie de estadísticas han venido a poner hoy en día más de manifiesto.