2014 Activity Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Conference on Rare Diseases
EUROPEAN CONFERENCE ON RARE DISEASES Luxembourg 21-22 June 2005 EUROPEAN CONFERENCE ON RARE DISEASES Copyright 2005 © Eurordis For more information: www.eurordis.org Webcast of the conference and abstracts: www.rare-luxembourg2005.org TABLE OF CONTENT_3 ------------------------------------------------- ACKNOWLEDGEMENTS AND CREDITS A specialised clinic for Rare Diseases : the RD TABLE OF CONTENTS Outpatient’s Clinic (RDOC) in Italy …………… 48 ------------------------------------------------- ------------------------------------------------- 4 / RARE, BUT EXISTING The organisers particularly wish to thank ACKNOWLEDGEMENTS AND CREDITS 4.1 No code, no name, no existence …………… 49 ------------------------------------------------- the following persons/organisations/companies 4.2 Why do we need to code rare diseases? … 50 PROGRAMME COMMITTEE for their role : ------------------------------------------------- Members of the Programme Committee ……… 6 5 / RESEARCH AND CARE Conference Programme …………………………… 7 …… HER ROYAL HIGHNESS THE GRAND DUCHESS OF LUXEMBOURG Key features of the conference …………………… 12 5.1 Research for Rare Diseases in the EU 54 • Participants ……………………………………… 12 5.2 Fighting the fragmentation of research …… 55 A multi-disciplinary approach ………………… 55 THE EUROPEAN COMMISSION Funding of the conference ……………………… 14 Transfer of academic research towards • ------------------------------------------------- industrial development ………………………… 60 THE GOVERNEMENT OF LUXEMBOURG Speakers ……………………………………………… 16 Strengthening cooperation between academia -

Regulations for Disease Reporting and Control
Department of Health Regulations for Disease Reporting and Control Commonwealth of Virginia State Board of Health October 2016 Virginia Department of Health Office of Epidemiology 109 Governor Street P.O. Box 2448 Richmond, VA 23218 Department of Health Department of Health TABLE OF CONTENTS Part I. DEFINITIONS ......................................................................................................................... 1 12 VAC 5-90-10. Definitions ............................................................................................. 1 Part II. GENERAL INFORMATION ............................................................................................... 8 12 VAC 5-90-20. Authority ............................................................................................... 8 12 VAC 5-90-30. Purpose .................................................................................................. 8 12 VAC 5-90-40. Administration ....................................................................................... 8 12 VAC 5-90-70. Powers and Procedures of Chapter Not Exclusive ................................ 9 Part III. REPORTING OF DISEASE ............................................................................................. 10 12 VAC 5-90-80. Reportable Disease List ....................................................................... 10 A. Reportable disease list ......................................................................................... 10 B. Conditions reportable by directors of -

LIST of OCCUPATIONAL DISEASES (Revised 2010)
LIST OF OCCUPATIONAL DISEASES (revised 2010) Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases Occupational Safety and Health Series, No. 74 List of occupational diseases (revised 2010) Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases INTERNATIONAL LABOUR OFFICE • GENEVA Copyright © International Labour Organization 2010 First published 2010 Publications of the International Labour Office enjoy copyright under Protocol 2 of the Universal Copyright Convention. Nevertheless, short excerpts from them may be reproduced without authorization, on condition that the source is indicated. For rights of reproduction or translation, application should be made to ILO Publications (Rights and Permissions), International Labour Office, CH-1211 Geneva 22, Switzerland, or by email: pubdroit@ ilo.org. The International Labour Office welcomes such applications. Libraries, institutions and other users registered with reproduction rights organizations may make copies in accordance with the licences issued to them for this purpose. Visit www.ifrro.org to find the reproduction rights organization in your country. ILO List of occupational diseases (revised 2010). Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases Geneva, International Labour Office, 2010 (Occupational Safety and Health Series, No. 74) occupational disease / definition. 13.04.3 ISBN 978-92-2-123795-2 ISSN 0078-3129 Also available in French: Liste des maladies professionnelles (révisée en 2010): Identification et reconnaissance des maladies professionnelles: critères pour incorporer des maladies dans la liste des maladies professionnelles de l’OIT (ISBN 978-92-2-223795-1, ISSN 0250-412x), Geneva, 2010, and in Spanish: Lista de enfermedades profesionales (revisada en 2010). -

ICD-10 International Statistical Classification of Diseases and Related Health Problems
ICD-10 International Statistical Classification of Diseases and Related Health Problems 10th Revision Volume 2 Instruction manual 2010 Edition WHO Library Cataloguing-in-Publication Data International statistical classification of diseases and related health problems. - 10th revision, edition 2010. 3 v. Contents: v. 1. Tabular list – v. 2. Instruction manual – v. 3. Alphabetical index. 1.Diseases - classification. 2.Classification. 3.Manuals. I.World Health Organization. II.ICD-10. ISBN 978 92 4 154834 2 (NLM classification: WB 15) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

FAQ REGARDING DISEASE REPORTING in MONTANA | Rev
Disease Reporting in Montana: Frequently Asked Questions Title 50 Section 1-202 of the Montana Code Annotated (MCA) outlines the general powers and duties of the Montana Department of Public Health & Human Services (DPHHS). The three primary duties that serve as the foundation for disease reporting in Montana state that DPHHS shall: • Study conditions affecting the citizens of the state by making use of birth, death, and sickness records; • Make investigations, disseminate information, and make recommendations for control of diseases and improvement of public health to persons, groups, or the public; and • Adopt and enforce rules regarding the reporting and control of communicable diseases. In order to meet these obligations, DPHHS works closely with local health jurisdictions to collect and analyze disease reports. Although anyone may report a case of communicable disease, such reports are submitted primarily by health care providers and laboratories. The Administrative Rules of Montana (ARM), Title 37, Chapter 114, Communicable Disease Control, outline the rules for communicable disease control, including disease reporting. Communicable disease surveillance is defined as the ongoing collection, analysis, interpretation, and dissemination of disease data. Accurate and timely disease reporting is the foundation of an effective surveillance program, which is key to applying effective public health interventions to mitigate the impact of disease. What diseases are reportable? A list of reportable diseases is maintained in ARM 37.114.203. The list continues to evolve and is consistent with the Council of State and Territorial Epidemiologists (CSTE) list of Nationally Notifiable Diseases maintained by the Centers for Disease Control and Prevention (CDC). In addition to the named conditions on the list, any occurrence of a case/cases of communicable disease in the 20th edition of the Control of Communicable Diseases Manual with a frequency in excess of normal expectancy or any unusual incident of unexplained illness or death in a human or animal should be reported. -

History of the Statistical Classification of Diseases and Causes of Death
Copyright information All material appearing in this report is in the public domain and may be reproduced or copied without permission; citation as to source, however, is appreciated. Suggested citation Moriyama IM, Loy RM, Robb-Smith AHT. History of the statistical classification of diseases and causes of death. Rosenberg HM, Hoyert DL, eds. Hyattsville, MD: National Center for Health Statistics. 2011. Library of Congress Cataloging-in-Publication Data Moriyama, Iwao M. (Iwao Milton), 1909-2006, author. History of the statistical classification of diseases and causes of death / by Iwao M. Moriyama, Ph.D., Ruth M. Loy, MBE, A.H.T. Robb-Smith, M.D. ; edited and updated by Harry M. Rosenberg, Ph.D., Donna L. Hoyert, Ph.D. p. ; cm. -- (DHHS publication ; no. (PHS) 2011-1125) “March 2011.” Includes bibliographical references. ISBN-13: 978-0-8406-0644-0 ISBN-10: 0-8406-0644-3 1. International statistical classification of diseases and related health problems. 10th revision. 2. International statistical classification of diseases and related health problems. 11th revision. 3. Nosology--History. 4. Death- -Causes--Classification--History. I. Loy, Ruth M., author. II. Robb-Smith, A. H. T. (Alastair Hamish Tearloch), author. III. Rosenberg, Harry M. (Harry Michael), editor. IV. Hoyert, Donna L., editor. V. National Center for Health Statistics (U.S.) VI. Title. VII. Series: DHHS publication ; no. (PHS) 2011- 1125. [DNLM: 1. International classification of diseases. 2. Disease-- classification. 3. International Classification of Diseases--history. 4. Cause of Death. 5. History, 20th Century. WB 15] RB115.M72 2011 616.07’8012--dc22 2010044437 For sale by the U.S. -

Neglected Diseases
Neglected Diseases What is a neglected disease? Why do we call these diseases neglected? How many people are affected by neglected diseases? What are some examples of neglected diseases? What can be done to prevent neglected diseases? How can neglected diseases be treated? Where can people get more information about neglected diseases? What is a neglected disease? Neglected diseases are conditions that inflict severe health burdens on the world’s poorest people. Many of these conditions are infectious diseases that are most prevalent in tropical climates, particularly in areas with unsafe drinking water, poor sanitation, substandard housing and little or no access to health care. Why do we call these diseases neglected? Diseases are said to be neglected if they are often overlooked by drug developers or by others instrumental in drug access, such as government officials, public health programs and the news media. Typically, private pharmaceutical companies cannot recover the cost of developing and producing treatments for these diseases. Another reason neglected diseases are not considered high priorities for prevention or treatment is because they usually do not affect people who live in the United States and other developed nations. Neglected diseases also lack visibility because they usually do not cause dramatic outbreaks that kill large numbers of people. Rather, such diseases usually exact their toll over a longer period of time, leading to crippling deformities, severe disabilities and/or relatively slow deaths. How many people are affected by neglected diseases? The World Health Organization (WHO) estimates that more than 1 billion people -- one- sixth of the world’s population -- suffer from one or more neglected diseases. -
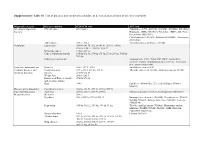
Supplementary Table S1. List of Diseases and Conditions Candidate to Be Tested As Predictors of One-Year Mortality
Supplementary Table S1. List of diseases and conditions candidate to be tested as predictors of one-year mortality Diagnostic category Disease/condition ICD-9 CM code ATC code Infectious and parasitic HIV infection 042.x-044.x Zidovudine (AZT) (J05AF01, J05AR01, J05AR04, J05AR05), diseases Didanosine (DDI) (J05AF02), Zalcitabine (DDC) (J05AF03), Pentamidine (P01CX01), Clarithromycin (J01FA09), Rifabutin (J04AB04), Atovaquone (P01AX06) Tuberculosis 010.x - 018.x Anti-tuberculosis antibiotics (J04AB) Neoplasms Lymphoma 200.00-202.38, 202.50-203.01, 203.8x, 238.6x, 273.3x, V10.71, V10.72, V10.79 Metastatic cancer 196.0x-199.1x Cancer, without metastasis 140.0x-172.9x, 174.0x-175.9x,179.x-195.8x, V10.0x- V10.9x Malignancy medication Antineoplastic (L01), Taxol (C07AB05), Interleukins (L03AC), Colony-stimulating factors (L03AA), Antinausea misc, ondansetron (A04) Endocrine, nutritional and Diabetes 250.x, 357.2, 362.0 Antidiabetic agents (A10) metabolic diseases, and Hypothyroidism 243.x-244.2, 244.8x, 244.9x Thyroid replacement (H03A), Antithyroid agents (H03B) immunity disorders Obesity 278.00-278.01 Weight loss 260.0x-263.9 Disorders of fluid, electrolyte, 276.0x-276.9x and acid-base balance Gout 274.x Colchicine (M04AC01), Uric acid inhibitors (M04AA, M04AB) Diseases of the blood and Coagulation defects 286.0x-286.9x, 287.1x, 287.3x-287.5x blood-forming organs Anaemias 280.0x, 280.1x-281.9x, 285.9x Marrow stimulants (L03AA), Erythropoietin (B03XA01) Mental disorders Dementia 290.x Psychosis 295.x-298.9x, 299.10-299.11 Butyrophenone derivates -
Detecting and Diagnosing Brain Diseases with Medical Imaging Table of Contents 3 Brainwatch Table of Contents
BRAINWATCH DETECTING AND diaGNOSING braiN diSEASES with MEdicaL IMAGING tabLE OF CONTENTS 3 brainwatch tabLE OF CONTENTS INTRODUCTIOn: What IS A NEUROradiOLOGIST? .................. 4 1 Imaging brain tumours: The neuroradiologist as the central chair on the brain tumour board .............................................................................9 2 Advanced imaging techniques in the diagnosis of dementia: from structure to function and back again ....................................................................................................................................17 3 Magnetic resonance imaging in the diagnosis of multiple sclerosis ...............................................................................................23 4 Magnetic resonance imaging in the diagnosis and treatment of Parkinson’s disease ......................................31 5 Radiotherapy of brain malignancies ...........................................39 6 Neuroimaging research ..................................................................................... 47 7 The patient’s view on brain imaging: European Federation of Neurological Associations .......................57 8 The Patient’s view on brain imaging: Austrian Self-Help Association ...........................................................................................65 9 Authors ..........................................................................................................................................71 photocredits ............................................................................................................................. -

Alzheimer Europe Annual Report 2011
Dementia in Europe Yearbook 2012 National Dementia Strategies (diagnosis, treatment and research) Including the Alzheimer Europe Annual Report 2011 The Dementia in Europe Yearbook arises from the 2012 Work Plan of Alzhei- mer Europe, which has received funding from the European Union, in the framework of the Health Programme. Neither the European Commission nor any person acting on its behalf is responsible for any use that might be made of the following information. Alzheimer Europe gratefully acknowledges the support it has received from the Alzheimer Europe Foundation for the publication of this Yearbook. 3 Table of contents Foreword ______________________________________________________________ 6 National Reports ________________________________________________________ 8 1 Austria _________________________________________________________ 9 2 Belgium _______________________________________________________ 11 3 Bulgaria _______________________________________________________ 13 4 Croatia ________________________________________________________ 17 5 Cyprus ________________________________________________________ 20 6 Czech Republic __________________________________________________ 22 7 Denmark ______________________________________________________ 26 8 Estonia _______________________________________________________ 31 9 Finland _______________________________________________________ 32 10 France ________________________________________________________ 36 11 Germany ______________________________________________________ 43 12 Greece -

A Classified List of Diseases and an Alphabetical List of the Causes of Death
Circular No. 34, A CLASSIFIED LIST OF DISEASES AND AN Alphabetical List of the Causes of Death. A CLASSIFIED LIST OF DISEASES AND AN Alphabetical List of the Causes of Death, Arranged to Refer One to the Other by Numbers, FOR THE USE OF REGISTRARS OF VITAL STATISTICS IN CONNECTICUT. With. Explanatory Remarks BY THE SUPERINTENDENT OF REGISTRATION. NEW HAVEN: TUTTLE, MOREHOUSE & TAYLOR, PRINTERS. 1887. To the Registrars of Births, Marriages and Deaths in the Towns of Connecticut: These few pages have been prepared for the use of the Regis- trars of Vital Statistics in the towns of Connecticut. The pur- pose is to afford them some ready help, in making the annual abstract of the deaths in their respective towns, which the law requires them to make and to send to the Secretary of the State Board of Health. By a recent legislation enacted in 1886, the town clerk of every town in the state, except New Haven, is made ex-officio the Registrar of Births, Marriages and Deaths for the town of which he is the Clerk. It is no reflection upon the intelligence of town clerks to say that they have not, and cannot have, as a consequence of being charged with new and unfamiliar duties, at once, the knowledge requisite to do well and correctly a portion of the work which the law imposes upon Registrars of Vital Statistics. Town <?lerks have not (with very few exceptions), theadvantages of a medical education. The varied and mixed systems of nam- ing diseases, in use among Doctors, are to almost all of them unknown. -

Recognition of Dementia in Hospitalized Older Adults
dementia series Best Practices in Nursing Care to Older Adults with dementia From The Hartford Institute for Geriatric Nursing, New York University, College of Nursing, and the Alzheimer’s Association Issue Number D5, Revised 2016 Editor-in-Chief: Sherry A. Greenberg, PhD, RN, GNP-BC New York University Rory Meyers College of Nursing Recognition of Dementia in Hospitalized Older Adults By: Mathy Mezey, EdD, RN, FAAN, NYU Rory Meyers College of Nursing and Katie Maslow, MSW, Gerontological Society of America WHY: About one fourth of older hospital patients have dementia (Maslow, 2006). Their dementia may never have been formally diagnosed, and even if it has been diagnosed, the diagnosis may not be noted in their hospital record. Because of stress caused by acute illness and being in an unfamiliar setting, some older patients show symptoms of dementia for the first time in the hospital. In addition, the stress of a hospitalization may worsen the cognition of people with Mild Cognitive Impairment (MCI) (Peterson, 2011). Older hospital patients with dementia are at much higher risk than other older hospital patients for delirium, falls, dehydration, inadequate nutrition, untreated pain, and medication-related problems. They are more likely to wander, to exhibit agitated and aggressive behaviors, to be physically restrained, and to experience functional decline that does not resolve following discharge. This Try This document suggests ways hospitals can increase recognition of dementia in their older patients, to lessen or avoid any of these problems. TARGET POPULATION: The prevalence of dementia in people 65 and over is between 10-20% (Peterson, 2011). Dementia should be considered a possibility in every hospital patient age 75 and over and can be present in younger patients as well.