Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Geometry Is the General Shape of a Molecule, As Determined by the Relative Positions of the Atomic Nuclei
Lecture Presentation Chapter 9 Molecular Geometries and Bonding Theories © 2012 Pearson Education, Inc. Chapter Goal • Lewis structures do not show shape and size of molecules. • Develop a relationship between two dimensional Lewis structure and three dimensional molecular shapes • Develop a sense of shapes and how those shapes are governed in large measure by the kind of bonds exist between the atoms making up the molecule © 2012 Pearson Education, Inc. Molecular geometry is the general shape of a molecule, as determined by the relative positions of the atomic nuclei. Copyright © Cengage Learning. All rights reserved. 10 | 3 The valence-shell electron-pair repulsion (VSEPR) model predicts the shapes of molecules and ions by assuming that the valence-shell electron pairs are arranged about each atom so that electron pairs are kept as far away from one another as possible, thereby minimizing electron pair repulsions. The diagram on the next slide illustrates this. Copyright © Cengage Learning. All rights reserved. 10 | 4 Two electron pairs are 180° apart (a linear arrangement). Three electron pairs are 120° apart in one plane (a trigonal planar arrangement). Four electron pairs are 109.5° apart in three dimensions (a tetrahedral arrangment). Copyright © Cengage Learning. All rights reserved. 10 | 5 Five electron pairs are arranged with three pairs in a plane 120° apart and two pairs at 90°to the plane and 180° to each other (a trigonal bipyramidal arrangement). Six electron pairs are 90° apart (an octahedral arrangement). This is illustrated on the next slide. Copyright © Cengage Learning. All rights reserved. 10 | 6 Copyright © Cengage Learning. All rights reserved. -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Valence Bond Theory
UMass Boston, Chem 103, Spring 2006 CHEM 103 Molecular Geometry and Valence Bond Theory Lecture Notes May 2, 2006 Prof. Sevian Announcements z The final exam is scheduled for Monday, May 15, 8:00- 11:00am It will NOT be in our regularly scheduled lecture hall (S- 1-006). The final exam location has been changed to Snowden Auditorium (W-1-088). © 2006 H. Sevian 1 UMass Boston, Chem 103, Spring 2006 More announcements Information you need for registering for the second semester of general chemistry z If you will take it in the summer: z Look for chem 104 in the summer schedule (includes lecture and lab) z If you will take it in the fall: z Look for chem 116 (lecture) and chem 118 (lab). These courses are co-requisites. z If you plan to re-take chem 103, in the summer it will be listed as chem 103 (lecture + lab). In the fall it will be listed as chem 115 (lecture) + chem 117 (lab), which are co-requisites. z Note: you are only eligible for a lab exemption if you previously passed the course. Agenda z Results of Exam 3 z Molecular geometries observed z How Lewis structure theory predicts them z Valence shell electron pair repulsion (VSEPR) theory z Valence bond theory z Bonds are formed by overlap of atomic orbitals z Before atoms bond, their atomic orbitals can hybridize to prepare for bonding z Molecular geometry arises from hybridization of atomic orbitals z σ and π bonding orbitals © 2006 H. Sevian 2 UMass Boston, Chem 103, Spring 2006 Molecular Geometries Observed Tetrahedral See-saw Square planar Square pyramid Lewis Structure Theory -

Sample Exercise 9.1 Using the VSEPR Model
Sample Exercise 9.1 Using the VSEPR Model – Use the VSEPR model to predict the molecular geometry of (a) O3, (b) SnCl3 . Solution Analyze: We are given the molecular formulas of a molecule and a polyatomic ion, both conforming to the general formula ABn and both having a central atom from the p block of the periodic table. Plan: To predict the molecular geometries of these species, we first draw their Lewis structures and then count the number of electron domains around the central atom. The number of electron domains gives the electron-domain geometry. We then obtain the molecular geometry from the arrangement of the domains that are due to bonds. (a) We can draw two resonance structures for O3: Because of resonance, the bonds between the central O atom and the outer O atoms are of equal length. In both resonance structures the central O atom is bonded to the two outer O atoms and has one nonbonding pair. Thus, there are three electron domains about the central O atoms. (Remember that a double bond counts as a single electron domain.) The arrangement of three electron domains is trigonal planar (Table 9.1). Two of the domains are from bonds, and one is due to a nonbonding pair. So, the molecule has a bent shape with an ideal bond angle of 120° (Table 9.2). Chemistry: The Central Science, Eleventh Edition Copyright ©2009 by Pearson Education, Inc. By Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, and Catherine J. Murphy Upper Saddle River, New Jersey 07458 With contributions from Patrick Woodward All rights reserved. -

Lewis Structure Handout
Lewis Structure Handout for Chemistry Students An electron dot diagram, also known as a Lewis Structure, is a representation of valence electrons in a single atom, and can be further utilized to depict the bonds that form based on the available electrons for covalent bonding between multiple atoms. -Each atom has a characteristic dot diagram based on its position in the periodic table and this reflects its potential for fulfillment of the octet rule. As a general rule, the noble gases have a filled octet (column VIII with eight valence electrons) and each preceding column has successively one fewer. • For example, Carbon is in column IV and has four valence electrons. It can therefore be represented as: • Each of these "lone" electrons can form a bond by sharing the orbital with another unbonded electron. Hydrogen, being in column I, has a single valence electron, and will therefore satisfy carbon's octet thusly: • When a compound has bonded electrons (as with CH4) it is most accurate to diagram it with lines representing each of the single bonds. • Using carbon again for a slightly more complicated example, it can also form double bonds. This is the case when carbon bonds to two oxygen atoms, resulting in CO2. The oxygen atoms (with six valence electrons in column VI) are each represented as: • Because carbon is the least electronegative of the atoms involved, it is placed centrally, and its valence electrons will migrate toward the more electronegative oxygens to form two double bonds and a linear structure. This can be written as: where oxygen's remaining lone pairs are still shown. -

Introduction to Molecular Orbital Theory
Chapter 2: Molecular Structure and Bonding Bonding Theories 1. VSEPR Theory 2. Valence Bond theory (with hybridization) 3. Molecular Orbital Theory ( with molecualr orbitals) To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond theory (with hybridization) and Molecular Orbital Theory. A good theory should predict physical and chemical properties of the molecule such as shape, bond energy, bond length, and bond angles.Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. The best it can do is suggest that these molecules are mixtures, or hybrids, of the two Lewis structures that can be written for these molecules. This problem, and many others, can be overcome by using a more sophisticated model of bonding based on molecular orbitals. Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. But this power carries a significant cost in terms of the ease with which the model can be visualized. One model does not describe all the properties of molecular bonds. Each model desribes a set of properties better than the others. The final test for any theory is experimental data. Introduction to Molecular Orbital Theory The Molecular Orbital Theory does a good job of predicting elctronic spectra and paramagnetism, when VSEPR and the V-B Theories don't. -
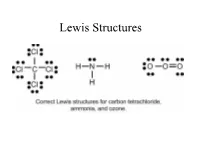
Lewis Structures
Lewis Structures Valence electrons for Elements Recall that the valence electrons for the elements can be determined based on the elements position on the periodic table. Lewis Dot Symbol Valence electrons and number of bonds Number of bonds elements prefers depending on the number of valence electrons. In general - F a m i l y → # C o v a l e n t B o n d s* H a l o g e n s X F , B r , C l , I → 1 bond often C a l c o g e n s O 2 bond often O , S → N i t r o g e n N 3 bond often N , P → C a r b o n C → 4 bond always C , S i The above chart is a guide on the number of bonds formed by these atoms. Lewis Structure, Octet Rule Guidelines When compounds are formed they tend to follow the Octet Rule. Octet Rule: Atoms will share electrons (e-) until it is surrounded by eight valence electrons. 4 unpaired 3unpaired 2unpaired 1unpaired up = unpaired e- 4 bonds 3 bonds 2 bonds 1 bond O=C=O N≡ N O = O F - F Atomic Connectivity The atomic arrangement for a molecule is usually given. CH2ClF HNO3 CH3COOH H2SO4 Cl N H O O O O H C F O H C C H O S O H H H H O H O In general when there is a single central atom in the 3- molecule, CH2ClF, SeCl2, O3 (CO2, NH3, PO4 ), the central atom is the first atom in the chemical formula. -

Draw Three Resonance Structures for the Chlorate Ion, Clo3
University Chemistry Quiz 3 2014/11/6 + 1. (10%) Explain why the bond order of N2 is greater than that of N2 , but the bond + order of O2 is less than that of O2 . Sol. + In forming the N2 from N2, an electron is removed from the sigma bonding molecular orbital. Consequently, the bond order decreases to 2.5 from 3.0. In + forming the O2 ion from O2, an electron is removed from the pi antibonding molecular orbital. Consequently, the bond order increases to 2.5 from 2.0. - 2. (10%) Draw three resonance structures for the chlorate ion, ClO3 . Show formal charges. Sol. Strategy: We follow the procedure for drawing Lewis structures outlined in Section 3.4 of the text. After we complete the Lewis structure, we draw the resonance structures. Solution: Following the procedure in Section 3.4 of the text, we come up with − the following Lewis structure for ClO3 . O − + − O Cl O We can draw two more equivalent Lewis structures with the double bond between Cl and a different oxygen atom. The resonance structures with formal charges are as follows: − − O O O + − − + − − + O Cl O O Cl O O Cl O 3. (5%) Use the molecular orbital energy-level diagram for O2 to show that the following Lewis structure corresponds to an excited state: Sol. The Lewis structure shows 4 pairs of electrons on the two oxygen atoms. From Table 3.4 of the text, we see that these 8 valence electrons are placed in the σ p p p p 2p , 2p , 2p , 2p , and 2p orbitals. -

Molecular Orbital Theory
Molecular Orbital Theory The Lewis Structure approach provides an extremely simple method for determining the electronic structure of many molecules. It is a bit simplistic, however, and does have trouble predicting structures for a few molecules. Nevertheless, it gives a reasonable structure for many molecules and its simplicity to use makes it a very useful tool for chemists. A more general, but slightly more complicated approach is the Molecular Orbital Theory. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. To understand MO Theory let's first review constructive and destructive interference of standing waves starting with the full constructive and destructive interference that occurs when standing waves overlap completely. When standing waves only partially overlap we get partial constructive and destructive interference. To see how we use these concepts in Molecular Orbital Theory, let's start with H2, the simplest of all molecules. The 1s orbitals of the H-atom are standing waves of the electron wavefunction. In Molecular Orbital Theory we view the bonding of the two H-atoms as partial constructive interference between standing wavefunctions of the 1s orbitals. The energy of the H2 molecule with the two electrons in the bonding orbital is lower by 435 kJ/mole than the combined energy of the two separate H-atoms. On the other hand, the energy of the H2 molecule with two electrons in the antibonding orbital is higher than two separate H-atoms. To show the relative energies we use diagrams like this: In the H2 molecule, the bonding and anti-bonding orbitals are called sigma orbitals (σ). -

Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Review Questions
Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Review Questions 10.1 J The properties of molecules are directly related to their shape. The sensation of taste, immune response, the sense of smell, and many types of drug action all depend on shape-specific interactions between molecules and proteins. According to VSEPR theory, the repulsion between electron groups on interior atoms of a molecule determines the geometry of the molecule. The five basic electron geometries are (1) Linear, which has two electron groups. (2) Trigonal planar, which has three electron groups. (3) Tetrahedral, which has four electron groups. (4) Trigonal bipyramid, which has five electron groups. (5) Octahedral, which has six electron groups. An electron group is defined as a lone pair of electrons, a single bond, a multiple bond, or even a single electron. H—C—H 109.5= ijj^^jl (a) Linear geometry \ \ (b) Trigonal planar geometry I Tetrahedral geometry I Equatorial chlorine Axial chlorine "P—Cl: \ Trigonal bipyramidal geometry 1 I Octahedral geometry I 369 370 Chapter 10 Chemical Bonding II The electron geometry is the geometrical arrangement of the electron groups around the central atom. The molecular geometry is the geometrical arrangement of the atoms around the central atom. The electron geometry and the molecular geometry are the same when every electron group bonds two atoms together. The presence of unbonded lone-pair electrons gives a different molecular geometry and electron geometry. (a) Four electron groups give tetrahedral electron geometry, while three bonding groups and one lone pair give a trigonal pyramidal molecular geometry. -
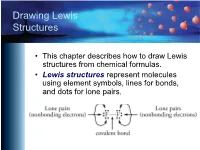
Drawing Lewis Structures
Drawing Lewis Structures • This chapter describes how to draw Lewis structures from chemical formulas. • Lewis structures represent molecules using element symbols, lines for bonds, and dots for lone pairs. Short Procedue for Drawing Lewis Structures • The first procedure involves drawing Lewis structures by attempting to give each atom in a molecule its most common bonding pattern. Most Common Bonding Patterns for Nonmetals Element # Bonds # lone pairs H 1 0 C 4 0 N, P 3 1 O, S, Se 2 2 F, Cl, Br, I 1 3 Most Common Bonding Patterns for Nonmetals https://preparatorychemistry.com/Bishop_periodic_table.pdf Example 1 Short Technique Methane, CH4 • Hydrogen atoms have 1 bond and no lone pairs. • Carbon atoms usually have 4 bonds and no lone pairs. Example 2 Short Technique Ammonia, NH3 • Hydrogen atoms have 1 bond and no lone pairs. • Nitrogen atoms usually have 3 bonds and 1 lone pair. Example 3 Short Technique Water, H2O • Hydrogen atoms have 1 bond and no lone pairs. • Oxygen atoms usually have 2 bonds and 2 lone pairs. Example 4 Short Technique Hypochlorous acid, HOCl • Hydrogen atoms have 1 bond and no lone pairs. • Oxygen atoms usually have 2 bonds and 2 lone pairs. • Chlorine atoms usually have one bond and three lone pairs. Example 5 Short Technique CFC-11, CCl3F • Carbon atoms usually have 4 bonds and no lone pairs. • Both fluorine and chlorine atoms usually have one bond and three lone pairs. Example 6 Short Technique Ethyne (acetylene), C2H2 (burned in oxyacetylene torches) • Carbon atoms usually have 4 bonds and no lone pairs. -

Chapter 9 Molecular Geometry and Bonding Theories
Secon 9.1 Hybridiza)on and the Localized Electron Model Chapter 9 Molecular Geometry and Bonding Theories Secon 9.1 Hybridiza)on and the Localized Electron Model VSEPR Model § VSEPR: Valence Shell Electron-Pair Repulsion. § The structure around a given atom is determined principally by minimizing electron pair repulsions. Copyright © Cengage Learning. All rights reserved 2 Secon 9.1 Hybridiza)on and the Localized Electron Model Steps to Apply the VSEPR Model 1. Draw the Lewis structure for the molecule. 2. Count the electron pairs and arrange them in the way that minimizes repulsion (put the pairs as far apart as possible. 3. Determine the posi$ons of the atoms from the way electron pairs are shared (how electrons are shared between the central atom and surrounding atoms). 4. Determine the name of the molecular structure from posi$ons of the atoms. Copyright © Cengage Learning. All rights reserved 3 Secon 9.1 Hybridiza)on and the Localized Electron Model Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB2 2 0 linear linear Copyright © Cengage Learning. All rights reserved Secon 9.1 Hybridiza)on and the Localized Electron Model # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry trigonal trigonal AB3 3 0 planar planar Copyright © Cengage Learning. All rights reserved Secon 9.1 Hybridiza)on and the Localized Electron Model # of atoms # lone bonded to pairs on Arrangement of Molecular Class central atom central atom electron pairs Geometry AB4 4 0 tetrahedral tetrahedral Copyright © Cengage Learning.