Digital Cranial Endocast of Hyopsodus (Mammalia, ‘‘Condylarthra’’): a Case of Paleogene Terrestrial Echolocation?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

Paleocene Mammalian Faunas of the Bison Basin in South-Central Wyoming
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 131, NUMBER 6 Cftarlesi ©, anb JHarp ^aux SKHalcott 3Res!earcf) Jf unb PALEOCENE MAMMALIAN FAUNAS OF THE BISON BASIN IN SOUTH-CENTRAL WYOMING (With 16 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology United States National Museum Smithsonian Institution (Publication 4229) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION FEBRUARY 28, 1956 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction i Acknowledgments 2 History of investigation 2 Occurrence and preservation of material 3 The Bison basin faunas 4 Environment and relationships between the Bison basin faunas 7 Age and correlation of the faunas lo Systematic description of vertebrate remains I2 Reptilia 12 Sauria 12 Anguidae 12 Mammalia 12 Multitubcrculata 12 Ptilodontidae 12 Marsupialia 14 Didelphidae 14 Insectivora 15 Leptictidae 15 Pantolestidae 17 Primates 19 Plesiadapidae 19 Carnivora 25 Arctocyonidae 25 Mesonychidae 35 Miacidae 35 Condylarthra 36 Hyopsodontidae 36 Phenacodontidae 42 Pantodonta 47 Coryphodontidae 47 References 51 Explanation of plates 54 ILLUSTRATIONS Plates (All plates following page 58.) 1. Mullituberculates and insectivores from the Bison basin Paleocene. 2. Primates and marsupials from the Bison basin Paleocene. 3. Pronothodcctes from the Bison basin Paleocene. 4. Plcsiadapis from the Bison basin Paleocene. 5. Triccntcs and Chriacus from the Bison basin Paleocene. 6. Thryptacodon from the Bison basin Paleocene. 7. Clacnodon from the Bison basin Paleocene. 8. Litomylus and Protoselcnc? from the Bison basin Paleocene. 9. Haplaletes and Gidleyina from the Bison basin Paleocene. 10. Phcnacodusl from the Bison basin Paleocene. 11. Condylarths and Titanoidcs from the Bison basin Paleocene. 12. Caenolambda from the Bison basin Paleocene. -

Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology
JOURNAL OF MORPHOLOGY 277:1469–1485 (2016) A Three-Dimensional Morphometric Analysis of Upper Forelimb Morphology in the Enigmatic Tapir (Perissodactyla: Tapirus) Hints at Subtle Variations in Locomotor Ecology Jamie A. MacLaren1* and Sandra Nauwelaerts1,2 1Department of Biology, Universiteit Antwerpen, Building D, Campus Drie Eiken, Universiteitsplein, Wilrijk, Antwerp 2610, Belgium 2Centre for Research and Conservation, Koninklijke Maatschappij Voor Dierkunde (KMDA), Koningin Astridplein 26, Antwerp 2018, Belgium ABSTRACT Forelimb morphology is an indicator for order Perissodactyla (odd-toed ungulates). Modern terrestrial locomotor ecology. The limb morphology of the tapirs are widely accepted to belong to a single enigmatic tapir (Perissodactyla: Tapirus) has often been genus (Tapirus), containing four extant species compared to that of basal perissodactyls, despite the lack (Hulbert, 1973; Ruiz-Garcıa et al., 1985) and sev- of quantitative studies comparing forelimb variation in eral regional subspecies (Padilla and Dowler, 1965; modern tapirs. Here, we present a quantitative assess- ment of tapir upper forelimb osteology using three- Wilson and Reeder, 2005): the Baird’s tapir (T. dimensional geometric morphometrics to test whether bairdii), lowland tapir (T. terrestris), mountain the four modern tapir species are monomorphic in their tapir (T. pinchaque), and the Malayan tapir (T. forelimb skeleton. The shape of the upper forelimb bones indicus). Extant tapirs primarily inhabit tropical across four species (T. indicus; T. bairdii; T. terrestris; T. rainforest, with some populations also occupying pinchaque) was investigated. Bones were laser scanned wet grassland and chaparral biomes (Padilla and to capture surface morphology and 3D landmark analysis Dowler, 1965; Padilla et al., 1996). was used to quantify shape. -

Origin and Beyond
EVOLUTION ORIGIN ANDBEYOND Gould, who alerted him to the fact the Galapagos finches ORIGIN AND BEYOND were distinct but closely related species. Darwin investigated ALFRED RUSSEL WALLACE (1823–1913) the breeding and artificial selection of domesticated animals, and learned about species, time, and the fossil record from despite the inspiration and wealth of data he had gathered during his years aboard the Alfred Russel Wallace was a school teacher and naturalist who gave up teaching the anatomist Richard Owen, who had worked on many of to earn his living as a professional collector of exotic plants and animals from beagle, darwin took many years to formulate his theory and ready it for publication – Darwin’s vertebrate specimens and, in 1842, had “invented” the tropics. He collected extensively in South America, and from 1854 in the so long, in fact, that he was almost beaten to publication. nevertheless, when it dinosaurs as a separate category of reptiles. islands of the Malay archipelago. From these experiences, Wallace realized By 1842, Darwin’s evolutionary ideas were sufficiently emerged, darwin’s work had a profound effect. that species exist in variant advanced for him to produce a 35-page sketch and, by forms and that changes in 1844, a 250-page synthesis, a copy of which he sent in 1847 the environment could lead During a long life, Charles After his five-year round the world voyage, Darwin arrived Darwin saw himself largely as a geologist, and published to the botanist, Joseph Dalton Hooker. This trusted friend to the loss of any ill-adapted Darwin wrote numerous back at the family home in Shrewsbury on 5 October 1836. -

Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming
Distribution and Stratigraphip Correlation of Upper:UB_ • Ju Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming U,S. GEOLOGICAL SURVEY PROFESS IONAL PAPER 1540 Cover. A member of the American Museum of Natural History 1896 expedition enter ing the badlands of the Willwood Formation on Dorsey Creek, Wyoming, near what is now U.S. Geological Survey fossil vertebrate locality D1691 (Wardel Reservoir quadran gle). View to the southwest. Photograph by Walter Granger, courtesy of the Department of Library Services, American Museum of Natural History, New York, negative no. 35957. DISTRIBUTION AND STRATIGRAPHIC CORRELATION OF UPPER PALEOCENE AND LOWER EOCENE FOSSIL MAMMAL AND PLANT LOCALITIES OF THE FORT UNION, WILLWOOD, AND TATMAN FORMATIONS, SOUTHERN BIGHORN BASIN, WYOMING Upper part of the Will wood Formation on East Ridge, Middle Fork of Fifteenmile Creek, southern Bighorn Basin, Wyoming. The Kirwin intrusive complex of the Absaroka Range is in the background. View to the west. Distribution and Stratigraphic Correlation of Upper Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Tatman Formations, Southern Bighorn Basin, Wyoming By Thomas M. Down, Kenneth D. Rose, Elwyn L. Simons, and Scott L. Wing U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1540 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsch, Acting Director For sale by U.S. Geological Survey, Map Distribution Box 25286, MS 306, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. -

Vertebrate Biostratigraphy of the Eocene Galisteo Formation, North-Central New Mexico Spencer G
New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/30 Vertebrate biostratigraphy of the Eocene Galisteo Formation, north-central New Mexico Spencer G. Lucas and Barry S. Kues, 1979, pp. 225-229 in: Santa Fe Country, Ingersoll, R. V. ; Woodward, L. A.; James, H. L.; [eds.], New Mexico Geological Society 30th Annual Fall Field Conference Guidebook, 310 p. This is one of many related papers that were included in the 1979 NMGS Fall Field Conference Guidebook. Annual NMGS Fall Field Conference Guidebooks Every fall since 1950, the New Mexico Geological Society (NMGS) has held an annual Fall Field Conference that explores some region of New Mexico (or surrounding states). Always well attended, these conferences provide a guidebook to participants. Besides detailed road logs, the guidebooks contain many well written, edited, and peer-reviewed geoscience papers. These books have set the national standard for geologic guidebooks and are an essential geologic reference for anyone working in or around New Mexico. Free Downloads NMGS has decided to make peer-reviewed papers from our Fall Field Conference guidebooks available for free download. Non-members will have access to guidebook papers two years after publication. Members have access to all papers. This is in keeping with our mission of promoting interest, research, and cooperation regarding geology in New Mexico. However, guidebook sales represent a significant proportion of our operating budget. Therefore, only research papers are available for download. Road logs, mini-papers, maps, stratigraphic charts, and other selected content are available only in the printed guidebooks. Copyright Information Publications of the New Mexico Geological Society, printed and electronic, are protected by the copyright laws of the United States. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Resolving the Relationships of Paleocene Placental Mammals
Biol. Rev. (2015), pp. 000–000. 1 doi: 10.1111/brv.12242 Resolving the relationships of Paleocene placental mammals Thomas J. D. Halliday1,2,∗, Paul Upchurch1 and Anjali Goswami1,2 1Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, U.K. 2Department of Genetics, Evolution and Environment, University College London, Gower Street, London WC1E 6BT, U.K. ABSTRACT The ‘Age of Mammals’ began in the Paleocene epoch, the 10 million year interval immediately following the Cretaceous–Palaeogene mass extinction. The apparently rapid shift in mammalian ecomorphs from small, largely insectivorous forms to many small-to-large-bodied, diverse taxa has driven a hypothesis that the end-Cretaceous heralded an adaptive radiation in placental mammal evolution. However, the affinities of most Paleocene mammals have remained unresolved, despite significant advances in understanding the relationships of the extant orders, hindering efforts to reconstruct robustly the origin and early evolution of placental mammals. Here we present the largest cladistic analysis of Paleocene placentals to date, from a data matrix including 177 taxa (130 of which are Palaeogene) and 680 morphological characters. We improve the resolution of the relationships of several enigmatic Paleocene clades, including families of ‘condylarths’. Protungulatum is resolved as a stem eutherian, meaning that no crown-placental mammal unambiguously pre-dates the Cretaceous–Palaeogene boundary. Our results support an Atlantogenata–Boreoeutheria split at the root of crown Placentalia, the presence of phenacodontids as closest relatives of Perissodactyla, the validity of Euungulata, and the placement of Arctocyonidae close to Carnivora. Periptychidae and Pantodonta are resolved as sister taxa, Leptictida and Cimolestidae are found to be stem eutherians, and Hyopsodontidae is highly polyphyletic. -

Vertebrate Paleontology of the Cretaceous/Tertiary Transition of Big Bend National Park, Texas (Lancian, Puercan, Mammalia, Dinosauria, Paleomagnetism)
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1986 Vertebrate Paleontology of the Cretaceous/Tertiary Transition of Big Bend National Park, Texas (Lancian, Puercan, Mammalia, Dinosauria, Paleomagnetism). Barbara R. Standhardt Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Standhardt, Barbara R., "Vertebrate Paleontology of the Cretaceous/Tertiary Transition of Big Bend National Park, Texas (Lancian, Puercan, Mammalia, Dinosauria, Paleomagnetism)." (1986). LSU Historical Dissertations and Theses. 4209. https://digitalcommons.lsu.edu/gradschool_disstheses/4209 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This reproduction was made from a copy of a manuscript sent to us for publication and microfilming. While the most advanced technology has been used to pho tograph and reproduce this manuscript, the quality of the reproduction is heavily dependent upon the quality of the material submitted. Pages in any manuscript may have indistinct print. In all cases the best available copy has been filmed. The following explanation of techniques is provided to help clarify notations which may appear on this reproduction. 1. Manuscripts may not always be complete. When it is not possible to obtain missing pages, a note appears to indicate this. 2. When copyrighted materials are removed from the manuscript, a note ap pears to indicate this. 3. -

Late Paleocene) of the Eastern Crazy Mountain Basin, Montana
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN VOL. 26, NO. 9, p. 157-196 December 3 1, 1983 MAMMALIAN FAUNA FROM DOUGLASS QUARRY, EARLIEST TIFFANIAN (LATE PALEOCENE) OF THE EASTERN CRAZY MOUNTAIN BASIN, MONTANA BY DAVID W. KRAUSE AND PHILIP D. GINGERICH MUSEUM OF PALEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Philip D. Gingerich, Director Gerald R. Smith, Editor This series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan, 48 109. VOLS. 11-XXVI. Parts of volumes may be obtained if available. Price lists available upon inquiry. MAMMALIAN FAUNA FROM DOUGLASS QUARRY, EARLIEST TIFFANIAN (LATE PALEOCENE) OF THE EASTERN CRAZY MOUNTAIN BASIN, MONTANA BY David W. ~rause'and Philip D. ~in~erich' Abstract.-Douglass Quarry is the fourth major locality to yield fossil mammals in the eastern Crazy Mountain Basin of south-central Montana. It is stratigraphically intermediate between Gidley and Silberling quarries below, which are late Torrejonian (middle Paleocene) in age, and Scarritt Quarry above, which is early Tiffanian (late Paleocene) in age. The stratigraphic position of Douglass Quarry and the presence of primitive species of Plesiadapis, Nannodectes, Phenacodus, and Ectocion (genera first appearing at the Torrejonian-Tiffanian boundary) combine to indicate an earliest Tiffanian age. -
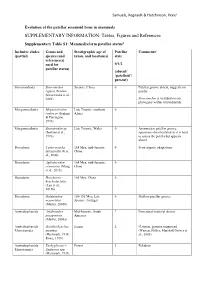
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -
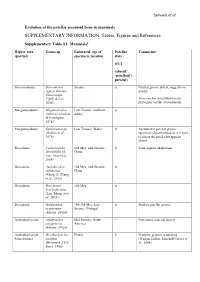
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp.