Aerosol Composition of Hot Giant Exoplanets Dominated by Silicates and Hydrocarbon Hazes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High Precision Photometry of Transiting Exoplanets
McNair Scholars Research Journal Volume 3 Article 3 2016 High Precision Photometry of Transiting Exoplanets Maurice Wilson Embry-Riddle Aeronautical University and Harvard-Smithsonian Center for Astrophysics Jason Eastman Harvard-Smithsonian Center for Astrophysics John Johnson Harvard-Smithsonian Center for Astrophysics Follow this and additional works at: https://commons.erau.edu/mcnair Recommended Citation Wilson, Maurice; Eastman, Jason; and Johnson, John (2016) "High Precision Photometry of Transiting Exoplanets," McNair Scholars Research Journal: Vol. 3 , Article 3. Available at: https://commons.erau.edu/mcnair/vol3/iss1/3 This Article is brought to you for free and open access by the Journals at Scholarly Commons. It has been accepted for inclusion in McNair Scholars Research Journal by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. Wilson et al.: High Precision Photometry of Transiting Exoplanets High Precision Photometry of Transiting Exoplanets Maurice Wilson1,2, Jason Eastman2, and John Johnson2 1Embry-Riddle Aeronautical University 2Harvard-Smithsonian Center for Astrophysics In order to increase the rate of finding, confirming, and characterizing Earth-like exoplanets, the MINiature Exoplanet Radial Velocity Array (MINERVA) was recently built with the purpose of obtaining the spectroscopic and photometric precision necessary for these tasks. Achieving the satisfactory photometric precision is the primary focus of this work. This is done with the four telescopes of MINERVA and the defocusing technique. The satisfactory photometric precision derives from the defocusing technique. The use of MINERVA’s four telescopes benefits the relative photometry that must be conducted. Typically, it is difficult to find satisfactory comparison stars within a telescope’s field of view when the primary target is very bright. -

Where Are the Distant Worlds? Star Maps
W here Are the Distant Worlds? Star Maps Abo ut the Activity Whe re are the distant worlds in the night sky? Use a star map to find constellations and to identify stars with extrasolar planets. (Northern Hemisphere only, naked eye) Topics Covered • How to find Constellations • Where we have found planets around other stars Participants Adults, teens, families with children 8 years and up If a school/youth group, 10 years and older 1 to 4 participants per map Materials Needed Location and Timing • Current month's Star Map for the Use this activity at a star party on a public (included) dark, clear night. Timing depends only • At least one set Planetary on how long you want to observe. Postcards with Key (included) • A small (red) flashlight • (Optional) Print list of Visible Stars with Planets (included) Included in This Packet Page Detailed Activity Description 2 Helpful Hints 4 Background Information 5 Planetary Postcards 7 Key Planetary Postcards 9 Star Maps 20 Visible Stars With Planets 33 © 2008 Astronomical Society of the Pacific www.astrosociety.org Copies for educational purposes are permitted. Additional astronomy activities can be found here: http://nightsky.jpl.nasa.gov Detailed Activity Description Leader’s Role Participants’ Roles (Anticipated) Introduction: To Ask: Who has heard that scientists have found planets around stars other than our own Sun? How many of these stars might you think have been found? Anyone ever see a star that has planets around it? (our own Sun, some may know of other stars) We can’t see the planets around other stars, but we can see the star. -

Analysis of New High-Precision Transit Light Curves of WASP-10 B: Starspot
Astronomy & Astrophysics manuscript no. wasp10 c ESO 2018 September 10, 2018 Analysis of new high-precision transit light curves of WASP-10 b: starspot occultations, small planetary radius, and high metallicity⋆ G. Maciejewski1,2, St. Raetz2, N.Nettelmann3, M. Seeliger2, Ch. Adam2, G. Nowak1, and R. Neuh¨auser2 1 Toru´nCentre for Astronomy, Nicolaus Copernicus University, Gagarina 11, PL–87100 Toru´n, Poland e-mail: [email protected] 2 Astrophysikalisches Institut und Universit¨ats-Sternwarte, Schillerg¨asschen 2–3, D–07745 Jena, Germany 3 Institut f¨ur Physik, Universit¨at Rostock, D–18051 Rostock, Germany Received ...; accepted ... ABSTRACT Context. The WASP-10 planetary system is intriguing because different values of radius have been reported for its transiting exoplanet. The host star exhibits activity in terms of photometric variability, which is caused by the rotational modulation of the spots. Moreover, a periodic modulation has been discovered in transit timing of WASP-10 b, which could be a sign of an additional body perturbing the orbital motion of the transiting planet. Aims. We attempt to refine the physical parameters of the system, in particular the planetary radius, which is crucial for studying the internal structure of the transiting planet. We also determine new mid-transit times to confirm or refute observed anomalies in transit timing. Methods. We acquired high-precision light curves for four transits of WASP-10 b in 2010. Assuming various limb-darkening laws, we generated best-fit models and redetermined parameters of the system. The prayer-bead method and Monte Carlo simulations were used to derive error estimates. -

A Hot Subdwarf-White Dwarf Super-Chandrasekhar Candidate
A hot subdwarf–white dwarf super-Chandrasekhar candidate supernova Ia progenitor Ingrid Pelisoli1,2*, P. Neunteufel3, S. Geier1, T. Kupfer4,5, U. Heber6, A. Irrgang6, D. Schneider6, A. Bastian1, J. van Roestel7, V. Schaffenroth1, and B. N. Barlow8 1Institut fur¨ Physik und Astronomie, Universitat¨ Potsdam, Haus 28, Karl-Liebknecht-Str. 24/25, D-14476 Potsdam-Golm, Germany 2Department of Physics, University of Warwick, Coventry, CV4 7AL, UK 3Max Planck Institut fur¨ Astrophysik, Karl-Schwarzschild-Straße 1, 85748 Garching bei Munchen¨ 4Kavli Institute for Theoretical Physics, University of California, Santa Barbara, CA 93106, USA 5Texas Tech University, Department of Physics & Astronomy, Box 41051, 79409, Lubbock, TX, USA 6Dr. Karl Remeis-Observatory & ECAP, Astronomical Institute, Friedrich-Alexander University Erlangen-Nuremberg (FAU), Sternwartstr. 7, 96049 Bamberg, Germany 7Division of Physics, Mathematics and Astronomy, California Institute of Technology, Pasadena, CA 91125, USA 8Department of Physics and Astronomy, High Point University, High Point, NC 27268, USA *[email protected] ABSTRACT Supernova Ia are bright explosive events that can be used to estimate cosmological distances, allowing us to study the expansion of the Universe. They are understood to result from a thermonuclear detonation in a white dwarf that formed from the exhausted core of a star more massive than the Sun. However, the possible progenitor channels leading to an explosion are a long-standing debate, limiting the precision and accuracy of supernova Ia as distance indicators. Here we present HD 265435, a binary system with an orbital period of less than a hundred minutes, consisting of a white dwarf and a hot subdwarf — a stripped core-helium burning star. -

Naming the Extrasolar Planets
Naming the extrasolar planets W. Lyra Max Planck Institute for Astronomy, K¨onigstuhl 17, 69177, Heidelberg, Germany [email protected] Abstract and OGLE-TR-182 b, which does not help educators convey the message that these planets are quite similar to Jupiter. Extrasolar planets are not named and are referred to only In stark contrast, the sentence“planet Apollo is a gas giant by their assigned scientific designation. The reason given like Jupiter” is heavily - yet invisibly - coated with Coper- by the IAU to not name the planets is that it is consid- nicanism. ered impractical as planets are expected to be common. I One reason given by the IAU for not considering naming advance some reasons as to why this logic is flawed, and sug- the extrasolar planets is that it is a task deemed impractical. gest names for the 403 extrasolar planet candidates known One source is quoted as having said “if planets are found to as of Oct 2009. The names follow a scheme of association occur very frequently in the Universe, a system of individual with the constellation that the host star pertains to, and names for planets might well rapidly be found equally im- therefore are mostly drawn from Roman-Greek mythology. practicable as it is for stars, as planet discoveries progress.” Other mythologies may also be used given that a suitable 1. This leads to a second argument. It is indeed impractical association is established. to name all stars. But some stars are named nonetheless. In fact, all other classes of astronomical bodies are named. -

Jahresbericht 1997 Zum Titelbild: Ein Neugeborener Stern (Kreuz), Tief in Den Staub Der Molekülwolke L1551 Eingebettet, Aus Der Er Entstand
Max-Planck-Institut für Astronomie Heidelberg-Königstuhl Jahresbericht 1997 Zum Titelbild: Ein neugeborener Stern (Kreuz), tief in den Staub der Molekülwolke L1551 eingebettet, aus der er entstand. Er ist nicht im Optischen, sondern nur als Infrarotquelle zu erkennen. Er emittiert in Polrichtung einen hellen Jet aus ioni- siertem Gas, beleuchtet einen ausgedehnten Reflexionsnebel, und regt die Herbig-Haro-Objekte HH 28 und HH 29 zum eigenen Leuchten an. Mehr dazu auf Seite 23–29. Max-Planck-Institut für Astronomie Heidelberg-Königstuhl Jahresbericht 1997 Das Max-Planck-Institut für Astronomie Geschäftsführende Direktoren: Prof. Dr. Steven Beckwith (bis 31. 8.), Prof. Dr. Immo Appenzeller (ab 1.8.1998) Wissenschaftliche Mitglieder, Kollegium, Direktoren: Prof. Dr. Immo Appenzeller (ab 1.8.1998, kommissarisch), Prof. Dr. Steven Beckwith, (ab 1. 9. 1998 beurlaubt), Prof. Dr. Hans Elsässer (bis 31. 3. 1997), Prof. Dr. Hans-Walter Rix (ab 1. 1. 1999). Fachbeirat: Prof. R. Bender, München; Prof. R.-J. Dettmar, Bochum; Prof. G. Hasinger, Potsdam; Prof. P. Léna, Meudon; Prof. M. Moles Villlamate, Madrid, Prof. F. Pacini, Firenze; Prof. K.-H. Schmidt, Potsdam; Prof. P.A. Strittmatter, Tuscon; Prof. S.D.M. White, Garching; Prof. L. Woltjer, St. Michel Obs. Derzeit hat das MPIA rund 160 Mitarbeiter, davon 43 Wissenschaftler, 37 Nachwuchs- und Gastwissenschaftler sowie 80 Techniker und Verwaltungsangestellte. Studenten der Fakultät für Physik und Astronomie der Universität Heidelberg führen am Institut Diplom- und Doktorarbeiten aus. In den Werkstätten des Instituts werden ständig Lehrlinge ausgebildet. Anschrift: MPI für Astronomie, Königstuhl 17, D-69117 Heidelberg. Telefon: 0049-6221-5280, Fax: 0049-6221-528246. E-mail: [email protected], Anonymous ftp: ftp.mpia-hd.mpg.de Homepage: http://www.mpia-hd.mpg.de Isophot Datacenter : [email protected]. -

The Extragalactic Distance Scale
The Extragalactic Distance Scale Published in "Stellar astrophysics for the local group" : VIII Canary Islands Winter School of Astrophysics. Edited by A. Aparicio, A. Herrero, and F. Sanchez. Cambridge ; New York : Cambridge University Press, 1998 Calibration of the Extragalactic Distance Scale By BARRY F. MADORE1, WENDY L. FREEDMAN2 1NASA/IPAC Extragalactic Database, Infrared Processing & Analysis Center, California Institute of Technology, Jet Propulsion Laboratory, Pasadena, CA 91125, USA 2Observatories, Carnegie Institution of Washington, 813 Santa Barbara St., Pasadena CA 91101, USA The calibration and use of Cepheids as primary distance indicators is reviewed in the context of the extragalactic distance scale. Comparison is made with the independently calibrated Population II distance scale and found to be consistent at the 10% level. The combined use of ground-based facilities and the Hubble Space Telescope now allow for the application of the Cepheid Period-Luminosity relation out to distances in excess of 20 Mpc. Calibration of secondary distance indicators and the direct determination of distances to galaxies in the field as well as in the Virgo and Fornax clusters allows for multiple paths to the determination of the absolute rate of the expansion of the Universe parameterized by the Hubble constant. At this point in the reduction and analysis of Key Project galaxies H0 = 72km/ sec/Mpc ± 2 (random) ± 12 [systematic]. Table of Contents INTRODUCTION TO THE LECTURES CEPHEIDS BRIEF SUMMARY OF THE OBSERVED PROPERTIES OF CEPHEID -

Ground-Based High Sensitivity Radio Astronomy at Decameter Wavelengths
"Planetary Radio Emissions IV" (Graz, 9/1996), H.O. Rucker et al. Eds., Austrian Acad. Sci. press, Vienna, p. 101-127, 1997 GROUND-BASED HIGH SENSITIVITY RADIO ASTRONOMY AT DECAMETER WAVELENGTHS Philippe Zarka1, Julien Queinnec1, Boris P. Ryabov2, Vladimir B. Ryabov2, Vyacheslav A. Shevchenko2, Alexei V. Arkhipov2, Helmut O. Rucker3 Laurent Denis4, Alain Gerbault4, Patrick Dierich5 and Carlo Rosolen5 1DESPA, CNRS/Observatoire de Paris, F-92195 Meudon, France 2Institute of RadioAstronomy, Krasnoznamennaya 4, Kharkov 310002, Ukraine 3Space Research Institute, Halbaerthgasse 1, Graz A-8010, Austria 4Station de RadioAstronomie de Nancay, USR B704, Observatoire de Paris, 18 Nançay, France 5ARPEGES, CNRS/Observatoire de Paris, F-92195 Meudon, France Abstract The decameter wave radio spectrum (~10-50 MHz) suffers a very high pollution by man- made interference. However, it is a range well-suited to the search for exoplanets and the study of planetary (especially Saturnian) lightning, provided that very high sensitivity (~1 Jansky) is available at high time resolution (0.1-1 second). Such conditions require a very large decameter radiotelescope and a broad, clean frequency band of observation. The latter can be achieved only through the use of a broadband multichannel-type receiving system, efficient data processing techniques for eliminating interference, and selection and integration of non-polluted frequency channels. This processing is then followed by an algorithm performing bursts detection and measuring their significance. These requirements have been met with the installation of an Acousto-Optical Spectrograph at the giant Ukrainian radiotelescope UTR-2, and four observations campaigns of Jupiter, Saturn, and the environment of nearby stars (including 51 Peg and 47 UMa) have been performed in 1995-96. -
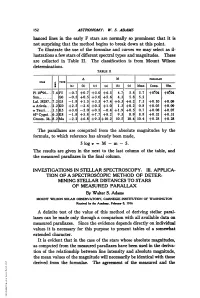
Not Surprising That the Method Begins to Break Down at This Point
152 ASTRONOMY: W. S. ADAMS hanced lines in the early F stars are normally so prominent that it is not surprising that the method begins to break down at this point. To illustrate the use of the formulae and curves we may select as il- lustrations a few stars of different spectral types and magnitudes. These are collected in Table II. The classification is from Mount Wilson determinations. TABLE H ~A M PARAIuAX srTATYP_________________ (a) I(b) (C) (a) (b) c) Mean Comp. Ob. Pi 10U96.... 7.6F5 -0.7 +0.7 +3.0 +6.5 4.7 5.8 5.7 +0#04 +0.04 Sun........ GO -0.5 +0.5 +3.0 +5.6 4.3 5.8 5.2 Lal. 38287.. 7.2 G5 -1.8 +1.5 +3.5 +7.4 +6.3 +6.2 7.3 +0.10 +0.09 a Arietis... 2.2K0O +2.5 -2.4 +0.2 +1.0 1.3 +0.2 0.8 +0.05 +0.09 aTauri.... 1.1K5 +3.0 -2.0 +0.5 -0.4 +1.9 +0.5 0.7 +0.08 +0.07 61' Cygni...6.3K8 -1.8 +5.8 +7.7 +8.2 9.3 8.9 8.8 +0.32 +0.31 Groom. 34..8.2 Ma -2.2 +6.8 +9.2 +10.2 10.5 10.4 10.4 +0.28 +0.28 The parallaxes are computed from the absolute magnitudes by the formula, to which reference has already been made, 5 logr = M - m - 5. The results are given in the next to the last column of the table, and the measured parallaxes in the final column. -

Open Batalha-Dissertation.Pdf
The Pennsylvania State University The Graduate School Eberly College of Science A SYNERGISTIC APPROACH TO INTERPRETING PLANETARY ATMOSPHERES A Dissertation in Astronomy and Astrophysics by Natasha E. Batalha © 2017 Natasha E. Batalha Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2017 The dissertation of Natasha E. Batalha was reviewed and approved∗ by the following: Steinn Sigurdsson Professor of Astronomy and Astrophysics Dissertation Co-Advisor, Co-Chair of Committee James Kasting Professor of Geosciences Dissertation Co-Advisor, Co-Chair of Committee Jason Wright Professor of Astronomy and Astrophysics Eric Ford Professor of Astronomy and Astrophysics Chris Forest Professor of Meteorology Avi Mandell NASA Goddard Space Flight Center, Research Scientist Special Signatory Michael Eracleous Professor of Astronomy and Astrophysics Graduate Program Chair ∗Signatures are on file in the Graduate School. ii Abstract We will soon have the technological capability to measure the atmospheric compo- sition of temperate Earth-sized planets orbiting nearby stars. Interpreting these atmospheric signals poses a new challenge to planetary science. In contrast to jovian-like atmospheres, whose bulk compositions consist of hydrogen and helium, terrestrial planet atmospheres are likely comprised of high mean molecular weight secondary atmospheres, which have gone through a high degree of evolution. For example, present-day Mars has a frozen surface with a thin tenuous atmosphere, but 4 billion years ago it may have been warmed by a thick greenhouse atmosphere. Several processes contribute to a planet’s atmospheric evolution: stellar evolution, geological processes, atmospheric escape, biology, etc. Each of these individual processes affects the planetary system as a whole and therefore they all must be considered in the modeling of terrestrial planets. -

Binocular Double Star Logbook
Astronomical League Binocular Double Star Club Logbook 1 Table of Contents Alpha Cassiopeiae 3 14 Canis Minoris Sh 251 (Oph) Psi 1 Piscium* F Hydrae Psi 1 & 2 Draconis* 37 Ceti Iota Cancri* 10 Σ2273 (Dra) Phi Cassiopeiae 27 Hydrae 40 & 41 Draconis* 93 (Rho) & 94 Piscium Tau 1 Hydrae 67 Ophiuchi 17 Chi Ceti 35 & 36 (Zeta) Leonis 39 Draconis 56 Andromedae 4 42 Leonis Minoris Epsilon 1 & 2 Lyrae* (U) 14 Arietis Σ1474 (Hya) Zeta 1 & 2 Lyrae* 59 Andromedae Alpha Ursae Majoris 11 Beta Lyrae* 15 Trianguli Delta Leonis Delta 1 & 2 Lyrae 33 Arietis 83 Leonis Theta Serpentis* 18 19 Tauri Tau Leonis 15 Aquilae 21 & 22 Tauri 5 93 Leonis OΣΣ178 (Aql) Eta Tauri 65 Ursae Majoris 28 Aquilae Phi Tauri 67 Ursae Majoris 12 6 (Alpha) & 8 Vul 62 Tauri 12 Comae Berenices Beta Cygni* Kappa 1 & 2 Tauri 17 Comae Berenices Epsilon Sagittae 19 Theta 1 & 2 Tauri 5 (Kappa) & 6 Draconis 54 Sagittarii 57 Persei 6 32 Camelopardalis* 16 Cygni 88 Tauri Σ1740 (Vir) 57 Aquilae Sigma 1 & 2 Tauri 79 (Zeta) & 80 Ursae Maj* 13 15 Sagittae Tau Tauri 70 Virginis Theta Sagittae 62 Eridani Iota Bootis* O1 (30 & 31) Cyg* 20 Beta Camelopardalis Σ1850 (Boo) 29 Cygni 11 & 12 Camelopardalis 7 Alpha Librae* Alpha 1 & 2 Capricorni* Delta Orionis* Delta Bootis* Beta 1 & 2 Capricorni* 42 & 45 Orionis Mu 1 & 2 Bootis* 14 75 Draconis Theta 2 Orionis* Omega 1 & 2 Scorpii Rho Capricorni Gamma Leporis* Kappa Herculis Omicron Capricorni 21 35 Camelopardalis ?? Nu Scorpii S 752 (Delphinus) 5 Lyncis 8 Nu 1 & 2 Coronae Borealis 48 Cygni Nu Geminorum Rho Ophiuchi 61 Cygni* 20 Geminorum 16 & 17 Draconis* 15 5 (Gamma) & 6 Equulei Zeta Geminorum 36 & 37 Herculis 79 Cygni h 3945 (CMa) Mu 1 & 2 Scorpii Mu Cygni 22 19 Lyncis* Zeta 1 & 2 Scorpii Epsilon Pegasi* Eta Canis Majoris 9 Σ133 (Her) Pi 1 & 2 Pegasi Δ 47 (CMa) 36 Ophiuchi* 33 Pegasi 64 & 65 Geminorum Nu 1 & 2 Draconis* 16 35 Pegasi Knt 4 (Pup) 53 Ophiuchi Delta Cephei* (U) The 28 stars with asterisks are also required for the regular AL Double Star Club. -

IAU Division C Working Group on Star Names 2019 Annual Report
IAU Division C Working Group on Star Names 2019 Annual Report Eric Mamajek (chair, USA) WG Members: Juan Antonio Belmote Avilés (Spain), Sze-leung Cheung (Thailand), Beatriz García (Argentina), Steven Gullberg (USA), Duane Hamacher (Australia), Susanne M. Hoffmann (Germany), Alejandro López (Argentina), Javier Mejuto (Honduras), Thierry Montmerle (France), Jay Pasachoff (USA), Ian Ridpath (UK), Clive Ruggles (UK), B.S. Shylaja (India), Robert van Gent (Netherlands), Hitoshi Yamaoka (Japan) WG Associates: Danielle Adams (USA), Yunli Shi (China), Doris Vickers (Austria) WGSN Website: https://www.iau.org/science/scientific_bodies/working_groups/280/ WGSN Email: [email protected] The Working Group on Star Names (WGSN) consists of an international group of astronomers with expertise in stellar astronomy, astronomical history, and cultural astronomy who research and catalog proper names for stars for use by the international astronomical community, and also to aid the recognition and preservation of intangible astronomical heritage. The Terms of Reference and membership for WG Star Names (WGSN) are provided at the IAU website: https://www.iau.org/science/scientific_bodies/working_groups/280/. WGSN was re-proposed to Division C and was approved in April 2019 as a functional WG whose scope extends beyond the normal 3-year cycle of IAU working groups. The WGSN was specifically called out on p. 22 of IAU Strategic Plan 2020-2030: “The IAU serves as the internationally recognised authority for assigning designations to celestial bodies and their surface features. To do so, the IAU has a number of Working Groups on various topics, most notably on the nomenclature of small bodies in the Solar System and planetary systems under Division F and on Star Names under Division C.” WGSN continues its long term activity of researching cultural astronomy literature for star names, and researching etymologies with the goal of adding this information to the WGSN’s online materials.