Quo Vadis Molecular Imaging?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Imaging – Introduction
MOLECULAR IMAGING – INTRODUCTION Author: Markus Rudin Affiliation: Institute for Biomedical Engineering, University and ETH Zurich, Institute for Pharmacology and Toxicology, University of Zurich, HCI-E488.2, ETH Zurich, CH-8093 Zurich, Switzerland, [email protected] Introduction The primary role of biomedical imaging is in diagnostics, i.e. the characterization of a disease phenotype based on morphological or physiological readouts. Image quality is rated based on two criteria: spatial resolution and contrast-to-noise ratio. Image contrast and thereby information can be enhanced by administration of labels that either enhance the contrast in the image or generate a signal that otherwise would not be detectable. When combining a signal generating moiety (a reporter group) with a target-specific carrier (receptor ligand, enzyme substrate, antibody, oligonucleotide, cell) highly specific information on targeted molecular interactions can be obtained. Such target-specific or molecular imaging approaches raised considerable interest both from a diagnostic and therapeutic point-of-view 1-3, and will become important tools for biomedical researchers. They provide temporo-spatial information on molecular processes in the intact organism, i.e. in the full biological context with all regulatory interactions in place. Molecular imaging techniques will therefore play a role for the validation for systems biological approaches. As for structural and functional imaging a primary application area of molecular imaging will be in diagnostics. Tissue/organ structure and function can be considered a phenotype of a molecular program. Being able to visualize and quantify these molecular processes will therefore provide early information on pathologies, eventually in a pre-morbid state. Molecular information will certainly enhance the accuracy of diagnosis allowing for better therapy planning for the individual patient (personalized medicine). -

Advanced Ultrasound Technologies for Diagnosis and Therapy
Journal of Nuclear Medicine, published on March 1, 2018 as doi:10.2967/jnumed.117.200030 1 Advanced Ultrasound Technologies for Diagnosis and Therapy Anne Rix1, Wiltrud Lederle1, Benjamin Theek1, Twan Lammers1,2, Chrit Moonen3, Georg Schmitz4, Fabian Kiessling1* 1Institute for Experimental Molecular Imaging, RWTH-Aachen University, Aachen, Germany 2Department of Targeted Therapeutics, University of Twente, Enschede, The Netherlands 3Imaging Division, University Medical Center Utrecht, Utrecht, The Netherland 4Department of Medical Engineering, Ruhr-University Bochum, Bochum, Germany * Corresponding author: Fabian Kiessling MD, Institute for Experimental Molecular Imaging, University Aachen (RWTH), Forckenbeckstrasse 55, 52074 Aachen, Germany. Phone:+49-241- 8080116; fax:+49-241-8082442; e-mail: [email protected] First author: Anne Rix B.Sc., Institute for Experimental Molecular Imaging, University Aachen (RWTH), Forckenbeckstrasse 55, 52074 Aachen, Germany. Phone:+49-241-8080839; fax:+49- 241-8082442; e-mail: [email protected] Running title Advanced Ultrasound Imaging and Therapy 1 2 ABSTRACT Ultrasound is among the most rapidly advancing imaging techniques. Functional methods such as elastography have been clinically introduced, and tissue characterization is improved by contrast- enhanced scans. Here, novel super-resolution techniques provide unique morphological and functional insights into tissue vascularisation. Functional analyses are complemented with molecular ultrasound imaging, to visualize markers of inflammation and angiogenesis. The full potential of diagnostic ultrasound may become apparent by integrating these multiple imaging features in radiomics approaches. Emerging interest in ultrasound also results from its therapeutic potential. Various applications on tumor ablation with high intensity focused ultrasound (HIFU) are clinically evaluated and its performance strongly benefits from the integration into Magnetic Resonance Imaging (MRI). -

Molecular Imaging Tutorial
This tutorial will define what is currently considered molecular imaging. It will provide history and an overview, discuss the goals and the advantages of molecular imaging. It will clarify what is and is not molecular imaging, and give examples of different imaging case studies. It will also discuss MRI, Optical Imaging, SPECT and PET imaging, and how they relate to molecular imaging. Molecular Imaging: Definition, Overview and Goals • Definition as published in Eur J Nucl Med: Molecular imaging has its roots in nuclear medicine and in many ways is a direct extension of this discipline. Nuclear Medicine has always focused on patient management through the use of injected radiolabeled tracers in conjunction with imaging technology. • Definition as published in Genes & Dev: Molecular imaging is the visual representation, characterization, and quantification of biological processes at the cellular and subcellular levels within intact living organisms. The images obtained reflect cellular and molecular pathways and in vivo mechanisms of disease present within the natural physiological environment. • Definition according to the American College of Radiology: Molecular imaging is the spatially localized and/or temporally resolved sensing of molecular and cellular processes in vivo. Overview of Molecular Imaging • Development of the first microscope in the late sixteenth century led to a keen interest in observations of structure of tissues and organs. This interest in organ structure and function has driven advances in biology ever since. • Molecular imaging originated from the field of radiopharmacology due to the need to better understand the fundamental molecular pathways inside organisms in a noninvasive manner. Molecular imaging unites molecular biology and in vivo imaging and enables the visualization of cellular function and the follow-up of the molecular process in living organisms without perturbing them. -

The Future Trends in Molecular Imaging
The Future Trends in Molecular Imaggging Dr. Juri Gelovani, MD , PhD . Professor of Radiology and Neurology Chairman, Dept. Experimental Diagnostic Imaging Director, Center for Advanced Biomedical Imaging Research (CABIR) Molecular And Genetic Imaging of Cancer El&AEarly & Accura tDite Diagnos is • To follow Biomarker Screening (blood genomics and proteomics) • Develop Imaging of Biomarkers (tumor localization, sensitivity) Tumor Phenotyping • Tumor Profiling (receptors & signal transduction, invasiveness, metabolism, proliferation, apoptosis, etc.) • Stroma Profiling (angiogenesis, tissue re-re-organization,organization, etc.) Imaging for Selection & Monitoring of Therapy • Drug Target Expression & Activity • Dose Optimization • Early Response Evaluation (Rx adjustment) • Shor t-t<Pterm & Long Term Prognos is • Monitoring Contained/Chronic Disease status • Early Detection of Relapse Image-Guided Biopsy, Surgery and Radiotherapy Image-Guided Biopsy Image-Guided Radiotherapy Sentinel lymph node mapping in breast carcinoma patients Sentinel lymph node mapping in breast carcinoma patients Imaging of angiogenesis in breast tumor SPECT imaging after injection of 99mTc-RGD peptide Potential for earlier assessment of response to chemotherapy • Avoid unnecessary cost and toxicity • Switch to alternative treatment sooner Approaches to Optical Imaging of Integrins in Neo-Angiogenesis SO 3H C(Gf)Cyclo(RGDfK)-CCy5.5 SO 3H Our pipeline: •MMP9 HO3S SO 3H •EGFR N N •PDGFR Gly • IL11Rα Arg Asp Lys phe O RGD + RGD-Cy5.5 Cy5. 5 RGD-C55Cy5.5 1 hr interval 6l/6 nmol/mouse 600 nmo l + 6 nmol 30 nmo l Structure of 111In-DTPA-K(IRDye800)-c(KRGDf). Gly Arg Asp O IRdye800 NH NH HN N H O O HN O H N O HOOC COOH NNN HOOC COOH COOH Gamma and near-infrared imaging of 111In-DTPA-K(IRDye800)-c(KRGDf) in nude mice bearing s.c. -

What Is Nuclear Medicine and Molecular Imaging?
What is Nuclear Medicine and Molecular Imaging? What is Nuclear Medicine and Molecular Imaging? The discovery of x-rays more than a century ago profoundly changed the practice of medicine by enabling physicians and scientists to see inside the living body. Today, modern medicine is undergoing another major transformation—and nuclear medicine and molecular imaging are on its leading edge, probing deep inside the body to reveal its inner workings. Unlike conventional imaging studies that produce primarily structural pictures, nuclear medicine and molecular imaging visualize how the body is functioning and what’s happening at the cellular and molecular level. The evolution in diagnostic imaging—from producing anatomical pictures to imaging and measuring the body’s physiological processes—is critically important to all facets of medicine today, from diagnosing disease at its earliest stage and developing more effective therapies to personalizing medical treatment. With the help of nuclear medicine and molecular imaging, scientists and healthcare providers are: Gaining a better understanding of the pathways of disease Quickly assessing new drugs Improving the selection of therapy Monitoring patient response to treatment Finding new ways to identify individuals at risk for disease. Why are Nuclear Medicine and Molecular Imaging unique? In conventional diagnostic imaging, an external source of energy such as x-rays, magnetic fields, or ultrasound waves is used to produce pictures of bone and soft tissue. In nuclear medicine and molecular imaging procedures, the energy source is introduced into the body, where it gets incorporated in a specific tissue, organ, or process and is then detected by an external device (gamma camera, SPECT or PET scanners) to provide information on organ function and cellular activity. -

Evicore Pelvis Imaging Guidelines
CLINICAL GUIDELINES Pelvis Imaging Policy Version 1.0 Effective February 14, 2020 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2017 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2019 eviCore healthcare. All rights reserved. Pelvis Imaging Guidelines V1.0 Pelvis Imaging Guidelines Abbreviations for Pelvis Imaging Guidelines 3 PV-1: General Guidelines 4 PV-2: Abnormal Uterine Bleeding 8 PV-3: Amenorrhea 10 PV-4: Adenomyosis 13 PV-5: Adnexal Mass/Ovarian Cysts 15 PV-6: Endometriosis 23 PV-7: Pelvic Inflammatory Disease (PID) 25 PV-8: Polycystic Ovary Syndrome 27 PV-9: Infertility Evaluation, Female 30 PV-10: Intrauterine Device (IUD) and Tubal Occlusion 32 PV-11: Pelvic Pain/Dyspareunia, Female 35 PV-12: Leiomyomata/Uterine Fibroids 38 PV-13: -

Contrast-Enhanced Ultrasonography: Advance and Current Status in Abdominal Imaging
Contrast-enhanced ultrasonography: advance and current status in abdominal imaging Yong Eun Chung, Ki Whang Kim REVIEW ARTICLE Department of Radiology, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea http://dx.doi.org/10.14366/usg.14034 pISSN: 2288-5919 • eISSN: 2288-5943 Ultrasonography 2015;34:3-18 In the field of contrast-enhanced ultrasonography (US), contrast agents are classified as either first- or second-generation agents depending on the gas within the microbubbles. In the case of Received: July 30, 2014 first-generation contrast agents, a high-mechanical-index technique is used and only intermittent Revised: September 10, 2014 Accepted: September 12, 2014 scanning is possible due to the early destruction of the microbubbles during the scanning. Correspondence to: The use of second-generation contrast agents in a low-mechanical-index technique enables Yong Eun Chung, MD, Department continuous scanning. Besides the detection and characterization of focal liver lesions, contrast- of Radiology, Research Institute of Radiological Science, Yonsei University enhanced US is helpful in the monitoring of radiofrequency ablation therapy and in the targeting College of Medicine, 50-1 Yonsei-ro, step of an US-guided biopsy. Recently, there has been a demand for new criteria to evaluate the Seodaemun-gu, Seoul 120-752, Korea treatment response obtained using anti-angiogenic agents because morphologic criteria alone Tel. +82-2-2228-7400 Fax. +82-2-393-3035 may not reflect the treatment response of the tumor and contrast-enhanced US can provide E-mail: [email protected] quantitative markers of tissue perfusion. In spite of the concerns related to its cost-effectiveness, *This paper is based on a previously contrast-enhanced US has the potential to be more widely used as a complimentary tool or to published review article entitled substitute the current imaging modalities in some occasions. -

Fluorescence Molecular Imaging
ANRV281-BE08-01 ARI 13 July 2006 2:2 Fluorescence Molecular Imaging Vasilis Ntziachristos Laboratory for Bio-Optics and Molecular Imaging, Center for Molecular Imaging Research, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114; email: [email protected] Annu. Rev. Biomed. Eng. Key Words 2006. 8:1–33 optical imaging, optical tomography, fluorescence, drug discovery, The Annual Review of Biomedical Engineering is small-animal imaging online at bioeng.annualreviews.org Abstract doi: 10.1146/ There is a wealth of new fluorescent reporter technologies for tagging of many cellular annurev.bioeng.8.061505.095831 and subcellular processes in vivo. This imposed contrast is now captured with an by b-on: Biblioteca do Conhecimento Online on 07/28/06. For personal use only. Annu. Rev. Biomed. Eng. 2006.8:1-33. Downloaded from arjournals.annualreviews.org Copyright c 2006 by increasing number of imaging methods that offer new ways to visualize and quantify Annual Reviews. All rights fluorescent markers distributed in tissues. This is an evolving field of imaging sciences reserved that has already achieved major advances but is also facing important challenges. It 1523-9829/06/0815- is nevertheless well poised to significantly impact the ways of biological research, 0001$20.00 drug discovery, and clinical practice in the years to come. Herein, the most pertinent technologies associated with in vivo noninvasive or minimally invasive fluorescence imaging of tissues are summarized. Focus is given to small-animal imaging. However, a spectrum of fluorescence reporters and imaging methods is outlined with broader potential applications to biomedical research and the clinical practice as well. -
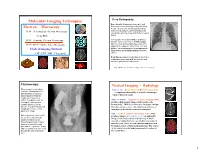
Molecular Imaging Techniques X-Ray Radiography Bone Absorbs X-Rays Particularly Well, Soft Tissue Has a Lower Density and Absorbs Fewer Electron Microscopy X-Rays
Molecular Imaging Techniques X-ray Radiography Bone absorbs X-rays particularly well, soft tissue has a lower density and absorbs fewer Electron Microscopy X-rays. Result is the familiar good contrast TEM – Transmission Electron Microscopy seen in X-ray images, with bones shown as clearly defined white areas and darker areas of tissue. (Cryo EM) SEM – Scanning Electron Microscopy Very suitable for scans of bones and tissue dense in calcium such as in dental images STM / AFM – Atomic Force Microsopy and detection of bone fractures, study of the organs in the abdomen such as the liver and Medical Imaging Methods bladder; chest radiography for pneumonia or lung cancer and mammography for breast cancer. (CAT / PET / MRI / Ultrasound) X-ray fluoroscopy is used to detect a number of diseases associated with the stomach and intestine, genitals and urinary tract. http://www.medicalradiation.com/types-of-medical-imaging/ Fluoroscopy: Medical Imaging - Radiology Fluoroscopy is used where CAT (or CT) - Computerized Axial Tomography real-time examination of the A computerized assembly of several x-ray images patient’s body is required. taken at different angles. positioning of orthopedic implants during surgery, catheters and pacemakers, MRI (or NMRI) - Magnetic resonance imaging (MRI) viewing the movement of produces high quality images of the inside of the contrast agents, such as human body. MRI is a noninvasive imaging technique barium, through the body and that does not use x-rays. The fluid contrast between studying the movement of structures in the brain can then be visualized. parts of the body. PET - positron emission tomography (PET); PET Barium products, taken orally, produces images of metabolic activity as opposed to are used for examining the images of the body's physical properties. -

Special Issue: Emerging Technologies for Medical Imaging Diagnostics, Monitoring and Therapy of Cancers
Journal of Clinical Medicine Editorial Special Issue: Emerging Technologies for Medical Imaging Diagnostics, Monitoring and Therapy of Cancers Mohsen Beheshti 1,* and Felix M. Mottaghy 2,3 1 Division of Molecular Imaging and Theranostics, University Hospital, Paracelsus Medical University, 5020 Salzburg, Austria 2 Department of Nuclear Medicine, University Hospital, RWTH University, 52074 Aachen, Germany; [email protected] 3 Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, 6229 Maastricht, The Netherlands * Correspondence: [email protected]; Tel.: +43-(0)5-7255-26602; Fax: +43-(0)5-7255-26699 Molecular imaging and therapy play an increasingly important role in the field of “precision medicine” as an emergent prospect for management of the cancerous disease. In this novel approach, individual variabilities such as the specific molecular biomarkers, genes, environment, and lifestyle of each person will be considered. This is, however, different from our current treatment concept, in which the prevention, diagnostic and therapy strategies are developed for a general population, with less focus on the distinctness between individuals. Our routine clinical practice of medicine was based on “personalized medicine” for decades, tailoring treatments to each patient. However, “precision medicine” considers the diagnostic and treatment approaches that are more precisely customized to specific molecular targets. In this era, the theranostic approach with targeted radionuclide therapy has unique promise in the precise management of cancers, as both the targeted Citation: Beheshti, M.; Mottaghy, vehicle and the radionuclide can be tailored to the individual patient. F.M. Special Issue: Emerging The present Special Issue in the Journal of Clinical Medicine aims to focus on novel Technologies for Medical Imaging advancements and future trends of molecular imaging and therapy as well as the thera- Diagnostics, Monitoring and Therapy nostic approach in the primary assessment and monitoring of disease in various cancers. -

Radiomic Analysis of Contrast-Enhanced Ultrasound Data
www.nature.com/scientificreports OPEN Radiomic analysis of contrast- enhanced ultrasound data Benjamin Theek1,2, Tatjana Opacic1, Zuzanna Magnuska1, Twan Lammers1,2 & Fabian Kiessling1,2 Received: 18 April 2018 Radiomics describes the use radiological data in a quantitative manner to establish correlations Accepted: 19 June 2018 in between imaging biomarkers and clinical outcomes to improve disease diagnosis, treatment Published: xx xx xxxx monitoring and prediction of therapy responses. In this study, we evaluated whether a radiomic analysis on contrast-enhanced ultrasound (CEUS) data allows to automatically diferentiate three xenograft mouse tumour models. Next to conventional imaging biomarker classes, i.e. intensity- based, textural, and wavelet-based features, we included biomarkers describing morphological and functional characteristics of the tumour vasculature. In total, 235 imaging biomarkers were extracted and evaluated. Dedicated feature selection allowed us to identify user-independent and stable imaging biomarkers for each imaging biomarker class. The selected radiomic signature, composed of median image intensity, energy of grey-level co-occurrence matrix, vessel network length, and run length nonuniformity of the grey-level run length matrix from the diagonal details, was used to train a linear support vector machine (SVM) to classify tumour phenotypes. The model was trained by using a four-fold cross-validation scheme and achieved 82.1% (95% CI [0.64 0.92]) correct classifcations. In conclusion, our results show that a radiomic analysis can be successfully performed on CEUS data and may help to render ultrasound-based tumour imaging more accurate, reproducible and reliable. Te huge geno- and phenotypic variability of tumours poses the question if better patient stratifcation and pre- cision medicine can help to improve the therapeutic outcome1–4. -

Applications in Molecular Ultrasound Imaging: Present and Future
Thomas Jefferson University Jefferson Digital Commons Department of Radiology Faculty Papers Department of Radiology 9-30-2019 Applications in Molecular Ultrasound Imaging: Present and Future Vishal Thumar, MD Lankenau Medical Center Ji-Bin Liu, MD Thomas Jefferson University John Eisenbrey, PhD Thomas Jefferson University Follow this and additional works at: https://jdc.jefferson.edu/radiologyfp Part of the Radiology Commons Let us know how access to this document benefits ouy Recommended Citation Thumar, MD, Vishal; Liu, MD, Ji-Bin; and Eisenbrey, PhD, John, "Applications in Molecular Ultrasound Imaging: Present and Future" (2019). Department of Radiology Faculty Papers. Paper 74. https://jdc.jefferson.edu/radiologyfp/74 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Radiology Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. REVIEW ARTICLE Applications in Molecular Ultrasound Imaging: Present and Future Vishal Thumar, MD a, Ji-Bin Liu, MD b, John Eisenbrey, PhD b,* a Department of Internal Medicine, Lankenau Medical Center, Wynnewood, PA, USA; b Department of Radiology, Thomas Jefferson University, Philadelphia, PA, USA Received May 7, 2019; revision received July 1; accepted July 6.