FEBS Open Bio (2020)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quantitative Portrait of Open Access Mega-Journals
Malaysian Journal of Library & Information Science, Vol. 24, no.2, August 2019: 115-131 Quantitative portrait of open access mega-journals Mohammadamin Erfanmanesh Department of Library and Information Science Faculty of Computer Science and Information Technology University of Malaya, Kuala Lumpur, MALAYSIA E-mail: [email protected]; [email protected] ABSTRACT Nowadays, Open Access Mega-Journals (OAMJs) represent a substantial part of the scholarly communication system. The current research is conducted with the aim of providing better insights into the increasingly important OAMJ phenomenon through investigation of eight reputable titles using established bibliometric methods. Results of the study showed that eight studied OAMJs were responsible for 1.87% of the total number of publication indexed in Web of Science during 2012-2016. Despite the decline in publication count of PLOS ONE over the past couple of years, it was the biggest journal in the world till 2017, when Scientific Reports overtook PLOS ONE as the most productive journal. Over 88% of the papers published in eight selected OAMJs were cited at-least once at the point in time of analysis. The highest proportions of cited and un-cited documents were seen in Scientific Reports and SpringerPlus, respectively. With regard to the three indicators, namely share of highly- cited papers, the category normalized citation impact as well as the JIF percentile, IEEE Access had by far the best performance among eight examined OAMJs. Results of the study revealed that Biochemistry & Molecular Biology, Multidisciplinary Sciences, Neurosciences, Oncology and Immunology were the most commonly assigned subject categories to OAMJs’ content. -

A Presentation of the Self-Publishing Model (SPM) As the Advent of a New Era of Scientific Communication
Self Publishing Model (SPM) A Presentation of the Self-Publishing Model (SPM) as the Advent of a New Era of Scientific Communication Author: Nour Ouda, Claus Jacob Endorsed by: Claus Jacob Edited by: Ahmad Yaman Abdin and Muhammad Jawad Nasim This manuscript is being published under the auspices of the Self Publishing Movement (SPM) to promote the free and unbiased exchange of scientific knowledge for all. The manuscript has not been refereed in order to protect the originality and to preserve the creativity of the author(s) and her/his/their ideas. Instead, it has been edited and endorsed by peers to ensure that format and content both adhere to acceptable international standards. Endorsements, criticism, comments and debate are always welcome. The intellectual property rights and copyright of this piece of original work reside with the author(s) and due credit should be given if this work is being cited. 0 Self Publishing Model (SPM) Abstract Traditional scientific dissemination via journals is problematic. It restricts the free exchange of knowledge due to financial restraints and, because of a subjective reviewing process, often impinges on authors’ originality. Eventually, important pieces of research are lost, whilst others are skewed in order to please anonymous referees. The Self-Publishing Movement (SPM) considers the free and open access to scientific knowledge as a right for all. Under the auspices of the SPM disseminating and acquiring knowledge is free of charge. The originality and creativity of authors’ work and ideas are preserved by avoiding the influence of referees. The standard linguistic and scientific quality of the work can be maintained by peer editors and endorsers before the dissemination. -

Author Guidelines Information About FEBS Open Bio
Author Guidelines Information about FEBS Open Bio .......................................................................................................... 1 Editorial Policies ............................................................................................................................................. 2 Types of manuscripts .................................................................................................................................... 4 Preparing your manuscript ....................................................................................................................... 5 After Acceptance .......................................................................................................................................... 12 Frequently asked questions ..................................................................................................................... 13 Information about FEBS Open Bio Aims and Scope FEBS Open Bio is an online-only open access journal for the rapid publication of research articles in molecular and cellular life sciences in both health and disease. The journal's peer review process focuses on the technical soundness of papers, leaving the assessment of their impact and importance to the scientific community. FEBS Open Bio publishes experimental findings, critical analysis, methodological and technical innovations, and hypotheses. Novel or innovative work is encouraged, but papers describing sound science of a confirmatory nature in developing fields or extending -

Issue 3 (November) 2014
ISSUE 3 (NOVEMBER) 2014 40th FEBS Congress FEBS programmes: FEBS–EMBO 2014 FEBS publications FEBS community updates conference round-up news Page 4 Page 8 Page 12 Page 26 Page 35 CONTENTS Contents: Key upcoming dates for Preface 3 FEBS activities FEBS Programmes: updates 40th FEBS Congress 40th FEBS Congress, Berlin, 2015 4 4–9 July 2015 FEBS Advanced Courses 8 Abstract submission deadline: 2 March 2015 FEBS Education Activities 11 Bursary application deadline: 2 March 2015 Early-bird registration deadline: 12 March 2015 FEBS–EMBO 2014 Conference Round-up www.febs2015.org The FEBS–EMBO 2014 Conference 12 FEBS Awards 14 FEBS Young Scientists’ Forum FEBS Workshops and Events 17 2–4 July 2014 FEBS Young Scientists’ Forum 2014 22 Application deadline: 31 January 2015 bit.ly/YSF2015 FEBS Fellows Meeting 2014 24 FEBS 50th Anniversary Dinner 25 FEBS Advanced Courses Applications for 2016 course funding: 1 March 2015 FEBS Publications Applications to participate in 2015 courses: see FEBS Publications at FEBS–EMBO 2014 26 individual course deadlines Digital Developments 28 www.febs.org/our-activities/advanced- Journal Highlights and Special Issues 33 courses/2015-advanced-courses FEBS Community News FEBS – Biochemical Society FEBS-sponsored Lectures 35 Education Workshop Obituary 38 FEBS Education Workshop bursaries deadline: FEBS Council: Elections 39 1 December 2014 Abstract submission deadline: 26 January 2015 Scientific Events Calendar 40 www.febs.org/our-activities/education Cover: Berlin, at the heart of the FEBS area, is the location for the 2015 FEBS Congress, hosted this time by the German Society for Biochemistry and Molecular Biology. Registration and abstract submission for the Congress have opened, and the event is introduced on pages 4–7 of this issue of FEBS News. -
Issue Information
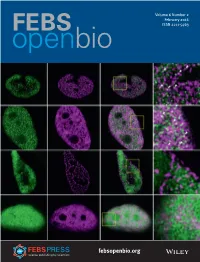
Volume 6 Number 2 February 2016 ISSN 2211-5463 febsopenbio.org FFEB4_v6_i2_IssueEB4_v6_i2_Issue info.inddinfo.indd 1 116-02-20166-02-2016 16:51:2516:51:25 EDITORIAL BOARD EDITORIAL OFFICE Senior Editors Laszlo Nagy Mary Purton, Executive Editor, FEBS Open Bio SBP Medical Discovery Institute, USA 98 Regent Street, Cambridge CB2 1DPUK Julio Celis and University of Debrecen, Hungary Institute of Cancer Biology, [email protected] Copenhagen, Denmark Zhen-Ming Pei Duke University, Durham, North Carolina, PRODUCTION OFFICE Stuart Ferguson USA University of Oxford, UK Marion Laws Jan S. Potempa Seamus Martin John Wiley & Sons Ltd, 9600 Garsington Road, Jagiellonian University Kraków, Poland Trinity College, Dublin, Ireland Oxford OX4 2DQ, UK Thomas Preiss [email protected] Felix Wieland Australian National University, Canberra, Ruprecht-Karls-Universität Heidelberg, Volume 6 Number 2 February 2016 Australia ISSN 1742-464X Germany Kristine Kleivi Sahlberg Associate Editors Oslo University Hospital, Norway Alberto Alape Giron Cristiano Simone Universidad de Costa Rica, San José, Università degli Studi di Bari Costa Rica Aldo Moro, Bari, Italy febsopenbio.org Edurne Berra Ramírez FEB4_v6_i2_oc.indd 1 16-02-2016 16:42:00 Sandro Sonnino CIC Biogune, DERIO, Spain Università degli Studi di Milano, Cover Illustration Antonio Castrillo Viguera Segrate, Italy In vivo fluorescence correlation spectroscopy analyses of FMBP-1, a silkworm transcription factor. From M. Tsutsumi et al. (pp. 106ă125). Instituto Investigaciones Biomedicas Michael Sussman Alberto Sols, Madrid, Spain University of Wisconsin at Madison, USA Pierre Cosson Tibor Vellai Université de Genève, Centre Medical Eötvös Lorand University, Budapest, Online ISSN: 2211-5463 Universitaire, Geneve 4-CH, Hungary This journal is Open Access; all articles will be immediately and Switzerland permanently free for everyone to read and download. -

(September) 2013
ISSUE 3 (SEPTEMBER) 2013 38th FEBS Congress FEBS programmes: FEBS community FEBS publications Scientific events round-up updates news calendar Page 3 Page 16 Page 20 Page 22 Page 27 CONTENTS Key upcoming dates for FEBS activities Contents: FEBS Congress Round-up FEBS–EMBO 2014 Conference 30 August – 4 September 2014 The 38th FEBS Congress 3 Abstract submission from December 2013 FEBS Medals and Awards 6 Registration from January 2014 FEBS Young Scientists’ Forum 8 www.febs-embo2014.org FEBS Congress Workshops 9 FEBS Congress Science and Society Lecture 12 FEBS Young Scientists’ Forum 27–30 August 2014 FEBS Programmes: updates Applications: 8 December 2013 – 31 March 2014 FEBS Advanced Courses 2014 16 www.febs-embo2014.org FEBS Education Workshops 17 FEBS–EMBO 2014 Conference, and FEBS YSF 18 FEBS Advanced Courses Applications for 2015 course funding: 1 March 2014 FEBS Community News Applications to participate in 2014 courses: see Spanish (SEBBM) Society 20 individual course deadlines Polish Biochemical Society 21 www.febs.org/index.php?id=86 FEBS Publications FEBS Fellowships FEBS Journal 22 Application deadlines: FEBS Letters 24 Long-Term and Return-to-Europe Fellowships: 1 October 2013, then 1 October 2014 Molecular Oncology 25 Summer Fellowships: 1 April 2014 FEBS Open Bio 26 Follow-up Research Fund (for FEBS Fellows): 1 April 2014 Applications for other Fellowships can be 27 Scientific Events Calendar submitted throughout the year Career Opportunities 28 www.febs.org/index.php?id=81 Cover: While this issue of FEBS News reports on the 2013 FEBS Congress in St Petersburg (pages 3–5), plans are already under way for next year’s big event – a joint anniversary conference with EMBO (the FEBS–EMBO 2014 Conference), hosted by the French Society for Biochemistry and Molecular Biology (see pages 18–19). -

Scopusindexedjournallis
Source Title Print-ISSN E-ISSN Open Acces status, i.e., registered in DOAJ and/or ROAD. Status March 2016 2D Materials 20531583 3 Biotech 2190572X 21905738 DOAJ/ROAD Open Access 3D Research 20926731 3L: Language, Linguistics, Literature 01285157 DOAJ/ROAD Open Access 4OR 16194500 16142411 A & A case reports 23257237 A + U-Architecture and Urbanism 03899160 A Contrario. Revue interdisciplinaire de sciences sociales 16607880 a/b: Auto/Biography Studies 21517290 A|Z ITU Journal of Faculty of Architecture 13028324 AAA, Arbeiten aus Anglistik und Amerikanistik 01715410 AAC: Augmentative and Alternative Communication 07434618 14773848 AACL Bioflux 18448143 18449166 DOAJ/ROAD Open Access AACN Advanced Critical Care 15597768 AANA Journal 00946354 AAO Journal 23755776 AAPG Bulletin 01491423 AAPP Atti della Accademia Peloritana dei Pericolanti, Classe di Scienze Fisiche, Matematiche e 03650359 18251242 DOAJ/ROAD Open Access Naturali AAPS Journal 15507416 AAPS PharmSciTech 15309932 15221059 DOAJ/ROAD Open Access AATCC Review 15328813 Ab Imperio 21649731 ABA Journal 07470088 Abacus 00013072 ABB Review 10133119 Abdominal Radiology 2366004X 23660058 Abhandlungen aus dem Mathematischen Seminar der Universitat Hamburg 00255858 Abstract and Applied Analysis 10853375 16870409 DOAJ/ROAD Open Access ABU Technical Review 01266209 Academe 01902946 Academia 10128255 Academic Emergency Medicine 10696563 Academic Journal of Manufacturing Engineering 15837904 Academic Journal of Second Military Medical University 0258879X Academic Medicine 10402446 Academic Pediatrics -

Publishing Volume • 1,000 New Editors Per Year • 20 New Journals Per Year • 600,000+ Article Submissions Per Year
How to write great papers and get them accepted in good journals From title to references From submission to acceptance Presented by: Anthony Newman Publisher, Elsevier, Amsterdam Location/Date: SOLABIAA, David, Panama 7 April 2013 Workshop Outline . How to get Published . Before you begin . Select your audience . The article structure . The review and editorial process . What not to do... (author ethics) 2 Peer –Reviewed Journal Growth 1665-2001 10000 100 Philosophical Transactions of the Royal Society (London) 2009 1,4 million articles in 23,000 journals by 2,000 publishers No of titles launched and still extant 2001 1 1665 1765 1865 1965 Source:Year M A Mabe The number and growth of journals 3 Serials 16(2).191-7, 2003 Elsevier Journal publishing volume • 1,000 new editors per year • 20 new journals per year • 600,000+ article submissions per year • Organise editorial boards Solicit and manage • 200,000 reviewers • Launch new specialist submissions • 1 million reviewer journals reports per year Archive and promote Manage peer review • 11 million articles • 40%-90% of now available articles rejected • 11 million • 7,000 editors researchers Publish and disseminate Edit and prepare • 70,000 editorial board • 5,000+ members institutions • 6.5 million • 180+ countries author/publisher • 400 million+ Production communications /year downloads per year • 3 million print • 280,000 new articles produced per year 4 pages4 per year • 190 years of back issues scanned, processed and data-tagged Trends in publishing . Rapid conversion from “print” to “electronic” . 1997: print only . 2009: 55% e-only (mostly e-collections) 25% print only 20% print-plus-electronic . -

FEBS Publications Committee Report 2013
FEBS Publications Committee Report 2013 In 2013 there were two Publications Committee Meetings: one in May (Cambridge) and one in July (St. Petersburg). The Chairman of the Committee attended the business meetings with Elsevier (Cambridge in March) and Wiley (Oxford in April); participated in the Editorial Board meetings of FEBS Letters (Debrecen in May) and FEBS Journal (Heidelberg in October); and was invited to join the Public Affairs Committee of “The Association of Learned and Professional Society Publishers” (ALPSP). The Committee decided that the fee for immediate open access would be the following from 2014: it stays at 3000 USD at FEBS Journal, and changes to 2200 USD in FEBS Letters and to 2700 USD in Molecular Oncology. (The Open Access publication fee for FEBS Open Bio is 1200 EUR.) The Committee appointed several new members to the editorial boards of the four FEBS journals. The Chairman of the Publications Committee, the chief editors of our journals and the journals themselves became signatories of DORA (The San Francisco “Declaration on Research Assessment “, DORA) which was made public on May 17 2013. The By-Laws of the Publications committee was modified effective by September 4, 2013. The new version of the By-Laws regulates clearly how elected/voting members of the Committee make decisions. The Publications Committee decided, after the limitation of the annual Prize money by FEBS Finance Committee to 5K for each Prize each year, that from the year 2015: - FEBS Letters Prize will be awarded every second year without age limitation, selecting the best paper and keeping the value of the prize money at 10K EUR; - FEBS Journal Prize will be awarded annually for young investigators without changing the established selection criteria but lowering the prize money significantly (very likely to 1000 EUR). -

St Petersburg 2013: 38Th FEBS Congress
ISSUE 1 (JANUARY) 2013 St Petersburg 2013: 38th FEBS Congress Mechanisms in Biology CONTENTS Contents: Key 2013 FEBS dates: Fellowships applications deadline Preface 3 1 April and 1 October 2013 (page 7) The 38th FEBS Congress 4 Advanced Courses applications deadline (for funding of 2014 courses) 1 April 2013 (page 8) FEBS Programmes: updates FEBS Fellowships news 7 Young Scientists’ Forum applications deadline FEBS Advanced Courses 2013 8 1 February 2013 (page 5 ) FEBS Education: recent workshops 11 Congress Bursary applications deadline Other upcoming events 13 10 March 2013 (page 5) FEBS Community News Congress early registration deadline 5 April 2013 (page 6) National Lectures 14 Hungarian Biochemical Society: 50 years 16 Congress registration deadline Obituary 19 1 June 2013 (page 6) 38th FEBS Congress FEBS Publications 6–11 July 2013 (pages 4–6) FEBS Journal 20 FEBS Letters 22 FEBS Advanced Courses Molecular Oncology 23 February–October 2013 (pages 8–10) (Registration deadlines are well ahead of course dates) FEBS Open Bio 23 Crick Memorial Meeting – 60th Anniversary Scientific Events Calendar 24 of DNA Structure 25 April 2013 (page 13) Career Opportunities 25 Education Workshops and FEBS 3+ Meeting July and autumn 2013 (page 13) Cover: St Petersburg, Russia, is the interesting location for the 38th FEBS Congress ’Mechanisms in Biology’, taking place from 6th to 11th July 2013. The cover photo shows the ‘Church of the Savior on Blood’, built from 1883 to 1907 in the style of medieval Russian architecture on the site where Tsar Alexander II was assassinated. Read more about the 2013 FEBS Congress on pages 4–7. -

Chemistry and Biochemistry Authored Faculty Publications
University of Arkansas, Fayetteville ScholarWorks@UARK University Libraries Faculty Publications and Presentations University Libraries 7-30-2020 Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR) Lutishoor Salisbury University of Arkansas, Fayetteville, [email protected] Yang Tian University of Arkansas, Fayetteville Jeremy Smith University of Arkansas, Fayetteville Follow this and additional works at: https://scholarworks.uark.edu/libpub Part of the Cataloging and Metadata Commons, Collection Development and Management Commons, Materials Chemistry Commons, and the Physical Chemistry Commons Citation Salisbury, L., Tian, Y., & Smith, J. (2020). Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR). University Libraries Faculty Publications and Presentations. Retrieved from https://scholarworks.uark.edu/libpub/38 This Report is brought to you for free and open access by the University Libraries at ScholarWorks@UARK. It has been accepted for inclusion in University Libraries Faculty Publications and Presentations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected]. University of Arkansas Libraries Chemistry and Biochemistry Library Chemistry and Biochemistry Authored Faculty Publications: Snapshot of the Ranking of Journals in which they published based on data from Journal Citation Reports (JCR). (https://go.openathens.net/redirector/uark.edu?url=https%3A%2F%2Fjcr.clarivate.com This report provides a snapshot of the ranking of journals for CHBC faculty authored papers for the period: 2015-2019. JCR is a database produced by Clarivate Analytics that provides impact factors and rankings of over 9000 journals. -

Open-Access Mega-Journals
Journal of Documentation Open-access mega-journals: The future of scholarly communication or academic dumping ground? A review Valerie Spezi Simon Wakeling Stephen Pinfield Claire Creaser Jenny Fry Peter Willett Article information: To cite this document: Valerie Spezi Simon Wakeling Stephen Pinfield Claire Creaser Jenny Fry Peter Willett , (2017)," Open-access mega-journals The future of scholarly communication or academic dumping ground? A review ", Journal of Documentation, Vol. 73 Iss 2 pp. 263 - 283 Permanent link to this document: http://dx.doi.org/10.1108/JD-06-2016-0082 Downloaded on: 17 March 2017, At: 02:47 (PT) References: this document contains references to 65 other documents. The fulltext of this document has been downloaded 1231 times since 2017* Users who downloaded this article also downloaded: (2017),"Resisting neoliberalism: the challenge of activist librarianship in English Higher Education", Journal of Documentation, Vol. 73 Iss 2 pp. 317-335 http://dx.doi.org/10.1108/JD-06-2016-0076 (2017),"Reading databases: slow information interactions beyond the retrieval paradigm", Journal of Documentation, Vol. 73 Iss 2 pp. 336-356 http://dx.doi.org/10.1108/JD-03-2016-0030 Access to this document was granted through an Emerald subscription provided by All users group For Authors If you would like to write for this, or any other Emerald publication, then please use our Emerald for Authors service information about how to choose which publication to write for and submission guidelines are available for all. Please visit www.emeraldinsight.com/authors for more information. Downloaded by University of Sheffield At 02:47 17 March 2017 (PT) About Emerald www.emeraldinsight.com Emerald is a global publisher linking research and practice to the benefit of society.