An Investigation Into the Function, Accuracy and Technology Associated with Arthroplasty of the Hip’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metal-On-Metal Hip Arthroplasty
METAL-ON-METAL HIP ARTHROPLASTY: INDICATIONS FOR CONTINUED USE IN LIGHT OF ADVERSE REACTION TO METAL DEBRIS by GULRAJ SINGH MATHARU A thesis submitted to the University of Birmingham for the degree of MASTERS OF RESEARCH IN CLINICAL RESEARCH School of Health and Population Sciences College of Medical and Dental Sciences University of Birmingham October 2014 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Adverse reaction to metal debris (ARMD) represents a recently recognised mode of failure of metal-on-metal (MoM) hip resurfacings (HRs) and total hip replacements (THRs). ARMD often requires revision surgery which can have poor outcomes, therefore questioning the future role of MoM hip bearings. The 14-year survival of 447 HRs implanted by a designing surgeon was 94.1% (95% CI 84.9%-97.3%) with the best outcomes in males with primary osteoarthritis. The 8-year survival of 578 MoM THRs was 88.9% (95% CI 78.5%-93.4%) with 44% (17 of 39) of revisions performed for ARMD. A systematic review identified six studies reporting short-term outcomes following 216 ARMD revisions with variable complication (4%-68%) and re-revision (3%-38%) rates. -
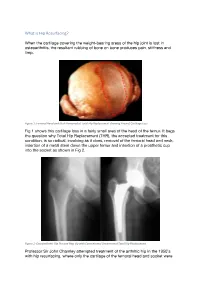
What Is Hip Resurfacing?
What is Hip Resurfacing? When the cartilage covering the weight-bearing areas of the hip joint is lost in osteoarthritis, the resultant rubbing of bone on bone produces pain, stiffness and limp. Figure 1: Femoral Head and Neck Removed at Total Hip Replacement showing Area of Cartilage Loss Fig 1 shows this cartilage loss in a fairly small area of the head of the femur. It begs the question why Total Hip Replacement (THR), the accepted treatment for this condition, is so radical, involving as it does, removal of the femoral head and neck, insertion of a metal stem down the upper femur and insertion of a prosthetic cup into the socket as shown in Fig 2. Figure 2: Osteoarthritic Hip Pre and Post Op with Conventional Uncemented Total Hip Replacement Professor Sir John Charnley attempted treatment of the arthritic hip in the 1950’s with hip resurfacing, where only the cartilage of the femoral head and socket were replaced with thin layers of polytetrafluoroethylene (PTFE). These implants unfortunately failed within months, Charnley thinking that the culprit was death of the femoral head as a result of interrupting its blood supply. He therefore moved on to a THR using PTFE cups paired with metal stemmed femoral components. Initially these were successful, and he carried out some 300 procedures. The initial success was not maintained, and at 2 years the implants started to fail with osteolysis [bone loss associated with the debris caused by wear]. Most of these patients needed further revision surgery, very distressing for them and their surgeon alike. -

1 Total Hip Replacement: Introduction, Sources and Outline
Notes 1 Total Hip Replacement: Introduction, Sources and Outline 1 ‘Railway Engineer’s Suicide’, The Times (9 May 1933). 2 Henderson, Melvin S. and Pollock, George A., ‘Surgical Treatment of Osteoarthritis of the Hip Joint’, Journal of Bone and Joint Surgery, 22 (1940), 923. 3 Cortisone was first synthesised in 1948 by the pharmaceutical company Merck. For the story of cortisone see Le Fanu, James, The Rise and Fall of Modern Medicine (London: Little, Brown and Company, 1999), 17–28. 4 Most of the early designs of these single component prostheses were devel- oped mainly in the US and in France. See Coventry, Mark B., ‘Lessons Learned in 30 Years of Total Hip Arthroplasty’, Clinical Orthopaedics and Related Research, 274 (1992), 22–9. 5 See Lerner, Barron H., The Breast Cancer Wars: Hope, fear and the pursuit of a cure in twentieth-century America (Oxford: Oxford University Press, 2001). For reflections on the role of surgery in different countries see Pickstone, John V., ‘Contested Cumulations: Configurations of cancer treatments through the twentieth century’, Bulletin of the History of Medicine, 81 (2007), 164–96. 6 For a history of TB in the twentieth century see Bryder, Linda, Below the Magic Mountain: A social history of tuberculosis in twentieth century Britain (Oxford: Oxford University Press, 1988). 7 Osteoarthritis is a degenerative condition, usually seen in older people and often affecting weight bearing joints in the lower limbs. See Cantor, David, ‘Representing “The Public”: Medicine, charity and emotion in twentieth century Britain’, in Sturdy, Steve (ed.), Medicine, Health and the Public Sphere in Britain, 1600–2000 (London: Routledge, 2002), 145–68. -

Early Development of Total Hip Replacement
EARLY DEVELOPMENT OF TOTAL HIP REPLACEMENT The transcript of a Witness Seminar held by the Wellcome Trust Centre for the History of Medicine at UCL, London, on 14 March 2006 Edited by L A Reynolds and E M Tansey Volume 29 2006 ©The Trustee of the Wellcome Trust, London, 2007 First published by the Wellcome Trust Centre for the History of Medicine at UCL, 2007 The Wellcome Trust Centre for the History of Medicine at UCL is funded by the Wellcome Trust, which is a registered charity, no. 210183. ISBN 978 085484 111 0 All volumes are freely available online at: www.history.qmul.ac.uk/research/modbiomed/wellcome_witnesses/ Please cite as: Reynolds L A, Tansey E M. (eds) (2007) Early Development of Total Hip Replacement. Wellcome Witnesses to Twentieth Century Medicine, vol. 29. London: Wellcome Trust Centre for the History of Medicine at UCL. CONTENTS Illustrations and credits v Abbreviations ix Witness Seminars: Meetings and publications; Acknowledgements E M Tansey and L A Reynolds xi Introduction Francis Neary and John Pickstone xxv Transcript Edited by L A Reynolds and E M Tansey 1 Appendix 1 Notes on materials by Professor Alan Swanson 95 Appendix 2 Surgical implant material standards by Mr Victor Wheble 97 Appendix 3 Selected prosthetic hips 101 References 107 Biographical notes 133 Glossary 147 Index 155 ILLUSTRATIONS AND CREDITS Figure 1 Site of a total hip transplant. Illustration provided by Ms Clare Darrah. 4 Figure 2 Mr Philip Wiles FRCS, c. 1950. Illustration provided by Sir Rodney Sweetnam. 5 Figure 3 X-ray of Wiles’ hip, c. -

RR Vol2no1august2012.Pdf
VOLUME 2 • NUMBER 1 August 2012 Reconstructive REVIEW OFFICIAL JOURNAL OF THE Joint Implant Surgery and Research Foundation Strategic Alliance with The Greenbrier Medical Institute Future Site Selected for Cutting-Edge Medical Initiative World Class Healthcare, Orthopaedics “Sports Medicine,” Rehabilitation, Plastic Surgery, Research & Education At America’s Greenbrier Resort White SulphurSprings, West Virginia Since 1948, the Greenbrier Clinic has been recognized as an industry leader in executive health and wellness through utilizing advanced diagnostics in the early diagnosis, prevention and treatment of disease. Building upon that history of medical excellence, Jim Justice, Chairman and owner of the Greenbrier Resort, has announced the creation of the Greenbrier Medical Institute. The institute’s 1st phase is projected to cost about $250 million, employ more than 500 people and include 3 buildings. This phase will include an expansion of our world renowned executive health and wellness practice, The Greenbrier Clinic, which will be bolstered by a world-class sports medicine program, including an orthopedic surgery center and athletic performance/rehabilitation facility, all led by the Founder of the American Sports Medicine Institute, Dr. Jim Andrews and Chair of Cleveland Clinic Innovations, Thomas Graham. Rounding out the Institute’s services will be a first-in-class plastic and cosmetic surgery and Lifestyle Enhancement Academy, helping people look and feel their best. Physicians, universities, research foundations, medical journals and other healthcare industry leaders, all of whom are on the cutting edge of medical technology, research and care, have committed to join the project and establish an international research and education destination or “think tank” to stimulate research, drive innovation, force change and redefine how the world approaches health, wellness and longevity. -

Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee Meeting
Joint Implant Surgery & Research Foundation Chagrin Falls, Ohio, USA Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee Meeting June 28, 2012 Metal (MoM) Bearings Questions and Discussions An Interview Facilitated by Timothy McTighe Timothy McTighe Dr. HS (hc) Advisory Committee Member: - Executive Director, JISRF - Publisher and Editor-in-Chief Michael B. Mayor, MD for Reconstructive Review, - William N. and Bessie Allyn JISRF Professor of Orthopaedic - Affiliate Member AAHKS Surgery, - Affiliate Member Mid- - The Geisel School of American Orthopaedic Medicine at Dartmouth association - Adjunct Professor of - Affiliate Member Australian Orthopaedic Engineering Association - Michael.B.Mayor@ - Member Orthopaedic Research Society Dartmouth.edu - Member Society of Biomaterials - Member International Society for Technology in Arthroplasty Presenter at Panel Meeting: Timothy McTighe was present on June 28, 2012 Bernard N. Stulberg, MD for the FDA Advisory Committee Meeting on Lutheran Hospital Metal -on- Metal Bearings. As part of the ongoing Cleveland, OH dialogue, JISRF has decided to conduct an interview Professional Societies on this day’s activities and publish this interview in - American Academy of its July edition of the Reconstruct Review. Orthopaedic Surgeons JISRF published an interview on May 31, 2010 on - American Orthopaedic the subject of MoM with eleven surgeons and their Association comments can be viewed at: http://www.jisrf.org/ - American Association of activities/052010.htm Hip and Knee Surgeons - Hip Society As we all know there is considerable debate and - Knee Society concern with the postoperative adverse reactions - International Society for Technology in that we are seeing world wide with the use of MoM Arthroplasty bearings. JISRF has conducted this interview with - Orthopaedic Research and Education six highly respected surgeons and one world class Foundation Tribologist based off the discussions held at the Special Interests recent FDA Device Panel Meeting. -

45Th Annual Meeting
Eastern Orthopaedic Association 45th Annual Meeting October 22-25, 2014 The Ritz-Carlton Amelia Island, FL Published by Data Trace Publishing Company Grantor & Exhibitor Acknowledgements The Eastern Orthopaedic Association is grateful for the support of its educational grantors and exhibitors. Thank you for your participation and commitment to EOA. Platinum Pacira Pharmaceuticals, Inc. Gold ConvaTec, Inc. Mallinckrodt Pharmaceuticals - Hospital Division Silver Zimmer, Inc. — Grantor Bronze Arthrex, Inc. — Grantor Smith & Nephew, Inc. Copper Blue Belt Technologies Innovative Medical Products, Inc. CeramTec Medical Products Integrity Rehab Group ConforMIS Marathon Pharmaceuticals, LLC DePuy Synthes MES Solutions DJO Global, Inc. MicroAire Surgical Instruments Exactech, Inc. National Surgical Healthcare Ferring Pharmaceuticals Inc. Quill / Surgical Specialities Corp. Exhibitors 3D Systems, Simbionix Medtronic Advanced Energy American Academy of Orthopaedic Surgeons MicroPort Orthopedics ACIGI Relaxation Modernizing Medicine, Inc. BBL Medical Facilities Nutramax Laboratories Customer Care, Inc. Biocomposites Ortho-Preferred Ceterix Orthopaedics Osiris Therapeutics, Inc. EOS imaging ProScan Reading Services Hospital Corporation of America Skeletal Dynamics iRemedy Terason LifeNet Health THINK Surgical Mallinckrodt Pharmaceuticals VirtaMed AG Medstrat Eastern Orthopaedic Association 45th Annual Meeting October 22-25, 2014 The Ritz-Carlton Amelia Island, Florida 2014 Meeting Program Chuck Freitag Executive Director, Data Trace Management Services, a Data Trace Company Cynthia Lichtefeld Director of Operations, Data Trace Management Services, a Data Trace Company EOA Central Offi ce, Data Trace Management Services 110 West Road, Suite 227 Towson, MD 21204 Phone: 866-362-1409 Fax: 410-494-0515 Email: [email protected] www.eoa-assn.org Please notify the EOA Central Offi ce of any changes in your home or offi ce address. -

2012 Meeting Program
Eastern Orthopaedic Association 43rd Annual Meeting June 20-23, 2012 The Sagamore on Lake George Bolton Landing, New York 2012 Meeting Program Chuck Freitag Executive Director, Data Trace Management Services, a Data Trace Company Cynthia Lichtefeld Director of Operations, Data Trace Management Services, a Data Trace Company EOA Central Office, Data Trace Management Services 110 West Road, Suite 227 Towson, MD 21204 Phone: 866-362-1409 Fax: 410-494-0515 Email: [email protected] www.eoa-assn.org Please notify the EOA Central Office of any changes in your home or office address. This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accred- itation Council for Continuing Medical Education (ACCME) through the joint Sponsorship of the American Acad- emy of Orthopaedic Surgeons and the Eastern Orthopaedic Association. The American Academy of Orthopaedic Surgeons is accredited by the ACCME to provide continuing medical education for physicians. The American Academy of Orthopaedic Surgeons designates this live activity for a maximum of 26.75 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity. Cover Design by Lauren Murphy. Copyright © 2012 by Data Trace Publishing Company, Towson, MD. All rights reserved. EOA 43rd Annual Meeting Bolton Landing, New York 2012 EOA President’s Message Dear EOA Colleagues: elcome to The Sagamore on Lake George at Bolton Landing. Thanks for attending the 43rd EOA Annual Meeting. Tara and I are delighted to be your host Wand hostess. The Sagamore staff are ready to make your stay enjoyable and com- fortable.