Elasticity and Equations of State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

Lecture 13: Earth Materials
Earth Materials Lecture 13 Earth Materials GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Hooke’s law of elasticity Force Extension = E × Area Length Hooke’s law σn = E εn where E is material constant, the Young’s Modulus Units are force/area – N/m2 or Pa Robert Hooke (1635-1703) was a virtuoso scientist contributing to geology, σ = C ε palaeontology, biology as well as mechanics ij ijkl kl ß Constitutive equations These are relationships between forces and deformation in a continuum, which define the material behaviour. GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Shear modulus and bulk modulus Young’s or stiffness modulus: σ n = Eε n Shear or rigidity modulus: σ S = Gε S = µε s Bulk modulus (1/compressibility): Mt Shasta andesite − P = Kεv Can write the bulk modulus in terms of the Lamé parameters λ, µ: K = λ + 2µ/3 and write Hooke’s law as: σ = (λ +2µ) ε GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Young’s Modulus or stiffness modulus Young’s Modulus or stiffness modulus: σ n = Eε n Interatomic force Interatomic distance GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Shear Modulus or rigidity modulus Shear modulus or stiffness modulus: σ s = Gε s Interatomic force Interatomic distance GNH7/GG09/GEOL4002 EARTHQUAKE SEISMOLOGY AND EARTHQUAKE HAZARD Hooke’s Law σij and εkl are second-rank tensors so Cijkl is a fourth-rank tensor. For a general, anisotropic material there are 21 independent elastic moduli. In the isotropic case this tensor reduces to just two independent elastic constants, λ and µ. -

Efficient Hydraulic Fluids Compressibility Is Still One of the Most Critically Important Factors
WORLDWIDE R. David Whitby Efficient hydraulic fluids Compressibility is still one of the most critically important factors. ydraulic fluids transmit power as a hydraulic system con- while phosphate and vegetable-oil esters have compressibilities H verts mechanical energy into fluid energy and subsequently similar to that of water. Polyalphaolefins are more compressible to mechanical work. To achieve this conversion efficiently, hydrau- than mineral oils. Water-glycol fluids are intermediate between lic fluids need to be relatively incompressible. Hydraulic fluids mineral oils and water. must also minimize wear, reduce friction, provide cooling and pre- Mineral oils are relatively incompressible, but volume reduc- vent rust and corrosion, be compatible with system components tions can be approximately 0.5% for pressures ranging from 6,900 and help keep the system free of deposits. kPa (1,000 psi) up to 27,600 kPa (4,000 psi). Compressibility in- Compressibility measures the rela- creases with pressure and temperature tive change in volume of a fluid or solid and has significant effects on high-pres- as a response to a change in pressure and sure fluid systems. Hydraulic oils typi- is the reciprocal of the volume-elastic cally contain 6% to 10% of dissolved air, modulus or bulk modulus of elasticity. which has no measurable effect on bulk Bulk modulus defines the pressure in- modulus provided it stays in solution. crease needed to cause a given relative Bulk modulus is an inherent property decrease in volume. of the hydraulic fluid and, therefore, is The bulk modulus of a fluid is nonlin- an inherent inefficiency of the hydraulic ear. -

Velocity, Density, Modulus of Hydrocarbon Fluids
Velocity, Density and Modulus of Hydrocarbon Fluids --Data Measurement De-hua Han*, HARC; M. Batzle, CSM Summary measured with pressure up to 55.2 Mpa (8000 Psi) and temperature up to 100 °C. Density and ultrasonic velocity of numerous hydrocarbon fluids (oil, oil based mud filtrate, hydrocarbon gases and Velocity of Dead Oil miscible CO2-oil) were measured at in situ conditions of pressure up to 50 Mpa and temperatures up to100 °C. Initial measurements were on the gas-free or ‘dead’ oils at Dynamic moduli are derived from velocities and densities. pressure and temperature (see Fig. 1). We used the Newly measured data refine correlations of velocity and following model to fit data: density to API gravity, Gas Oil ratio (GOR), Gas gravity and in situ pressure and temperature. Gas in solution is Vp (m/s) = A – B * T + C * P + D * T * P (1) largely responsible for reducing the bulk modulus of the live oil. Phase changes, such as exsolving gas during Here A is a pseudo velocity at 0 °C and room pressure (0 production, can dramatically lower velocities and modulus, Mpa, gauge), B is temperature gradient, C is pressure but is dependent on pressure conditions. Distinguish gas gradient and D is coefficient of coupled temperature and from liquid phase may not be possible at a high pressure. pressure effects. Fluids are often supercritical. With increasing pressure, a gas-like fluid can begin to behave like a liquid Dead & live oil of #1 B.P. = 1900 psi at 60 C Introduction 1800 1600 Hydrocarbon fluids are the primary targets of the seismic exploration. -
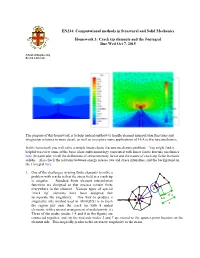
Crack Tip Elements and the J Integral
EN234: Computational methods in Structural and Solid Mechanics Homework 3: Crack tip elements and the J-integral Due Wed Oct 7, 2015 School of Engineering Brown University The purpose of this homework is to help understand how to handle element interpolation functions and integration schemes in more detail, as well as to explore some applications of FEA to fracture mechanics. In this homework you will solve a simple linear elastic fracture mechanics problem. You might find it helpful to review some of the basic ideas and terminology associated with linear elastic fracture mechanics here (in particular, recall the definitions of stress intensity factor and the nature of crack-tip fields in elastic solids). Also check the relations between energy release rate and stress intensities, and the background on the J integral here. 1. One of the challenges in using finite elements to solve a problem with cracks is that the stress field at a crack tip is singular. Standard finite element interpolation functions are designed so that stresses remain finite a everywhere in the element. Various types of special b c ‘crack tip’ elements have been designed that 3L/4 incorporate the singularity. One way to produce a L/4 singularity (the method used in ABAQUS) is to mesh L the region just near the crack tip with 8 noded elements, with a special arrangement of nodal points: (i) Three of the nodes (nodes 1,4 and 8 in the figure) are connected together, and (ii) the mid-side nodes 2 and 7 are moved to the quarter-point location on the element side. -

Elastic Properties of Fe−C and Fe−N Martensites
Elastic properties of Fe−C and Fe−N martensites SOUISSI Maaouia a, b and NUMAKURA Hiroshi a, b * a Department of Materials Science, Osaka Prefecture University, Naka-ku, Sakai 599-8531, Japan b JST-CREST, Gobancho 7, Chiyoda-ku, Tokyo 102-0076, Japan * Corresponding author. E-mail: [email protected] Postal address: Department of Materials Science, Graduate School of Engineering, Osaka Prefecture University, Gakuen-cho 1-1, Naka-ku, Sakai 599-8531, Japan Telephone: +81 72 254 9310 Fax: +81 72 254 9912 1 / 40 SYNOPSIS Single-crystal elastic constants of bcc iron and bct Fe–C and Fe–N alloys (martensites) have been evaluated by ab initio calculations based on the density-functional theory. The energy of a strained crystal has been computed using the supercell method at several values of the strain intensity, and the stiffness coefficient has been determined from the slope of the energy versus square-of-strain relation. Some of the third-order elastic constants have also been evaluated. The absolute magnitudes of the calculated values for bcc iron are in fair agreement with experiment, including the third-order constants, although the computed elastic anisotropy is much weaker than measured. The tetragonally distorted dilute Fe–C and Fe–N alloys exhibit lower stiffness than bcc iron, particularly in the tensor component C33, while the elastic anisotropy is virtually the same. Average values of elastic moduli for polycrystalline aggregates are also computed. Young’s modulus and the rigidity modulus, as well as the bulk modulus, are decreased by about 10 % by the addition of C or N to 3.7 atomic per cent, which agrees with the experimental data for Fe–C martensite. -

Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems
NfST Nisr National institute of Standards and Technology Technology Administration, U.S. Department of Commerce NIST Special Publication 946 Guide to Rheological Nomenclature: Measurements in Ceramic Particulate Systems Vincent A. Hackley and Chiara F. Ferraris rhe National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering, and develops measurement techniques, test methods, standards, and related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Making and Characterizing Negative Poisson's Ratio Materials
Making and characterizing negative Poisson’s ratio materials R. S. LAKES* and R. WITT**, University of Wisconsin–Madison, 147 Engineering Research Building, 1500 Engineering Drive, Madison, WI 53706-1687, USA. 〈[email protected]〉 Received 18th October 1999 Revised 26th August 2000 We present an introduction to the use of negative Poisson’s ratio materials to illustrate various aspects of mechanics of materials. Poisson’s ratio is defined as minus the ratio of transverse strain to longitudinal strain in simple tension. For most materials, Poisson’s ratio is close to 1/3. Negative Poisson’s ratios are counter-intuitive but permissible according to the theory of elasticity. Such materials can be prepared for classroom demonstrations, or made by students. Key words: Poisson’s ratio, deformation, foam, honeycomb 1. INTRODUCTION Poisson’s ratio is the ratio of lateral (transverse) contraction strain to longitudinal extension strain in a simple tension experiment. The allowable range of Poisson’s ratio ν in three 1 dimensional isotropic solids is from –1 to 2 [1]. Common materials usually have a Poisson’s 1 1 ratio close to 3 . Rubbery materials, however, have values approaching 2 . They readily undergo shear deformations, governed by the shear modulus G, but resist volumetric (bulk) deformation governed by the bulk modulus K; for rubbery materials G Ӷ K. Even though textbooks can still be found [2] which categorically state that Poisson’s ratios less than zero are unknown, or even impossible, there are in fact a number of examples of negative Poisson’s ratio solids. Although such behaviour is counter-intuitive, negative Poisson’s ratio structures and materials can be easily made and used in lecture demonstrations and for student projects. -

Chapter 7 – Geomechanics
Chapter 7 GEOMECHANICS GEOTECHNICAL DESIGN MANUAL January 2019 Geotechnical Design Manual GEOMECHANICS Table of Contents Section Page 7.1 Introduction ....................................................................................................... 7-1 7.2 Geotechnical Design Approach......................................................................... 7-1 7.3 Geotechnical Engineering Quality Control ........................................................ 7-2 7.4 Development Of Subsurface Profiles ................................................................ 7-2 7.5 Site Variability ................................................................................................... 7-2 7.6 Preliminary Geotechnical Subsurface Exploration............................................. 7-3 7.7 Final Geotechnical Subsurface Exploration ...................................................... 7-4 7.8 Field Data Corrections and Normalization ......................................................... 7-4 7.8.1 SPT Corrections .................................................................................... 7-4 7.8.2 CPTu Corrections .................................................................................. 7-7 7.8.3 Correlations for Relative Density From SPT and CPTu ....................... 7-10 7.8.4 Dilatometer Correlation Parameters .................................................... 7-11 7.9 Soil Loading Conditions And Soil Shear Strength Selection ............................ 7-12 7.9.1 Soil Loading ....................................................................................... -

Determination of Shear Modulus of Dental Materials by Means of the Torsion Pendulum
NATIONAL BUREAU OF STANDARDS REPORT 10 068 Progress Report on DETERMINATION OF SHEAR MODULUS OF DENTAL MATERIALS BY MEANS OF THE TORSION PENDULUM U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS NATIONAL BUREAU OF STANDARDS The National Bureau of Standards ' was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation’s central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation’s scientific community, industry, and com- merce. The Institute consists of an Office of Measurement Services -

Chapter 14 Solids and Fluids Matter Is Usually Classified Into One of Four States Or Phases: Solid, Liquid, Gas, Or Plasma
Chapter 14 Solids and Fluids Matter is usually classified into one of four states or phases: solid, liquid, gas, or plasma. Shape: A solid has a fixed shape, whereas fluids (liquid and gas) have no fixed shape. Compressibility: The atoms in a solid or a liquid are quite closely packed, which makes them almost incompressible. On the other hand, atoms or molecules in gas are far apart, thus gases are compressible in general. The distinction between these states is not always clear-cut. Such complicated behaviors called phase transition will be discussed later on. 1 14.1 Density At some time in the third century B.C., Archimedes was asked to find a way of determining whether or not the gold had been mixed with silver, which led him to discover a useful concept, density. m ρ = V The specific gravity of a substance is the ratio of its density to that of water at 4oC, which is 1000 kg/m3=1 g/cm3. Specific gravity is a dimensionless quantity. 2 14.2 Elastic Moduli A force applied to an object can change its shape. The response of a material to a given type of deforming force is characterized by an elastic modulus, Stress Elastic modulus = Strain Stress: force per unit area in general Strain: fractional change in dimension or volume. Three elastic moduli will be discussed: Young’s modulus for solids, the shear modulus for solids, and the bulk modulus for solids and fluids. 3 Young’s Modulus Young’s modulus is a measure of the resistance of a solid to a change in its length when a force is applied perpendicular to a face. -

FORMULAS for CALCULATING the SPEED of SOUND Revision G
FORMULAS FOR CALCULATING THE SPEED OF SOUND Revision G By Tom Irvine Email: [email protected] July 13, 2000 Introduction A sound wave is a longitudinal wave, which alternately pushes and pulls the material through which it propagates. The amplitude disturbance is thus parallel to the direction of propagation. Sound waves can propagate through the air, water, Earth, wood, metal rods, stretched strings, and any other physical substance. The purpose of this tutorial is to give formulas for calculating the speed of sound. Separate formulas are derived for a gas, liquid, and solid. General Formula for Fluids and Gases The speed of sound c is given by B c = (1) r o where B is the adiabatic bulk modulus, ro is the equilibrium mass density. Equation (1) is taken from equation (5.13) in Reference 1. The characteristics of the substance determine the appropriate formula for the bulk modulus. Gas or Fluid The bulk modulus is essentially a measure of stress divided by strain. The adiabatic bulk modulus B is defined in terms of hydrostatic pressure P and volume V as DP B = (2) - DV / V Equation (2) is taken from Table 2.1 in Reference 2. 1 An adiabatic process is one in which no energy transfer as heat occurs across the boundaries of the system. An alternate adiabatic bulk modulus equation is given in equation (5.5) in Reference 1. æ ¶P ö B = ro ç ÷ (3) è ¶r ø r o Note that æ ¶P ö P ç ÷ = g (4) è ¶r ø r where g is the ratio of specific heats.