How the Mammalian Endoplasmic Reticulum Handles Aggregation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Computational Genome-Wide Identification of Heat Shock Protein Genes in the Bovine Genome [Version 1; Peer Review: 2 Approved, 1 Approved with Reservations]](https://docslib.b-cdn.net/cover/8283/computational-genome-wide-identification-of-heat-shock-protein-genes-in-the-bovine-genome-version-1-peer-review-2-approved-1-approved-with-reservations-88283.webp)
Computational Genome-Wide Identification of Heat Shock Protein Genes in the Bovine Genome [Version 1; Peer Review: 2 Approved, 1 Approved with Reservations]
F1000Research 2018, 7:1504 Last updated: 08 AUG 2021 RESEARCH ARTICLE Computational genome-wide identification of heat shock protein genes in the bovine genome [version 1; peer review: 2 approved, 1 approved with reservations] Oyeyemi O. Ajayi1,2, Sunday O. Peters3, Marcos De Donato2,4, Sunday O. Sowande5, Fidalis D.N. Mujibi6, Olanrewaju B. Morenikeji2,7, Bolaji N. Thomas 8, Matthew A. Adeleke 9, Ikhide G. Imumorin2,10,11 1Department of Animal Breeding and Genetics, Federal University of Agriculture, Abeokuta, Nigeria 2International Programs, College of Agriculture and Life Sciences, Cornell University, Ithaca, NY, 14853, USA 3Department of Animal Science, Berry College, Mount Berry, GA, 30149, USA 4Departamento Regional de Bioingenierias, Tecnologico de Monterrey, Escuela de Ingenieria y Ciencias, Queretaro, Mexico 5Department of Animal Production and Health, Federal University of Agriculture, Abeokuta, Nigeria 6Usomi Limited, Nairobi, Kenya 7Department of Animal Production and Health, Federal University of Technology, Akure, Nigeria 8Department of Biomedical Sciences, Rochester Institute of Technology, Rochester, NY, 14623, USA 9School of Life Sciences, University of KwaZulu-Natal, Durban, 4000, South Africa 10School of Biological Sciences, Georgia Institute of Technology, Atlanta, GA, 30032, USA 11African Institute of Bioscience Research and Training, Ibadan, Nigeria v1 First published: 20 Sep 2018, 7:1504 Open Peer Review https://doi.org/10.12688/f1000research.16058.1 Latest published: 20 Sep 2018, 7:1504 https://doi.org/10.12688/f1000research.16058.1 Reviewer Status Invited Reviewers Abstract Background: Heat shock proteins (HSPs) are molecular chaperones 1 2 3 known to bind and sequester client proteins under stress. Methods: To identify and better understand some of these proteins, version 1 we carried out a computational genome-wide survey of the bovine 20 Sep 2018 report report report genome. -

Gene Targeting Therapies (Roy Alcalay)
Recent Developments in Gene - Targeted Therapies for Parkinson’s Disease Roy Alcalay, MD, MS Alfred and Minnie Bressler Associate Professor of Neurology Division of Movement Disorders Columbia University Medical Center Disclosures Funding: Dr. Alcalay is funded by the National Institutes of Health, the DOD, the Michael J. Fox Foundation and the Parkinson’s Foundation. Dr. Alcalay receives consultation fees from Genzyme/Sanofi, Restorbio, Janssen, and Roche. Gene Localizations Identified in PD Gene Symbol Protein Transmission Chromosome PARK1 SNCA α-synuclein AD 4q22.1 PARK2 PRKN parkin (ubiquitin ligase) AR 6q26 PARK3 ? ? AD 2p13 PARK4 SNCA triplication α-synuclein AD 4q22.1 PARK5 UCH-L1 ubiquitin C-terminal AD 4p13 hydrolase-L1 PARK6 PINK1 PTEN-induced kinase 1 AR 1p36.12 PARK7 DJ-1 DJ-1 AR 1p36.23 PARK8 LRRK2 leucine rich repeat kinase 2 AD 12q12 PARK9 ATP13A2 lysosomal ATPase AR 1p36.13 PARK10 ? ? (Iceland) AR 1p32 PARK11 GIGYF2 GRB10-interacting GYF protein 2 AD 2q37.1 PARK12 ? ? X-R Xq21-q25 PARK13 HTRA2 serine protease AD 2p13.1 PARK14 PLA2G6 phospholipase A2 (INAD) AR 22q13.1 PARK15 FBXO7 F-box only protein 7 AR 22q12.3 PARK16 ? Discovered by GWAS ? 1q32 PARK17 VPS35 vacuolar protein sorting 35 AD 16q11.2 PARK18 EIF4G1 initiation of protein synth AD 3q27.1 PARK19 DNAJC6 auxilin AR 1p31.3 PARK20 SYNJ1 synaptojanin 1 AR 21q22.11 PARK21 DNAJC13 8/RME-8 AD 3q22.1 PARK22 CHCHD2 AD 7p11.2 PARK23 VPS13C AR 15q22 Gene Localizations Identified in PD Disorder Symbol Protein Transmission Chromosome PD GBA β-glucocerebrosidase AD 1q21 SCA2 -

Identification and Characterization of TPRKB Dependency in TP53 Deficient Cancers
Identification and Characterization of TPRKB Dependency in TP53 Deficient Cancers. by Kelly Kennaley A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Molecular and Cellular Pathology) in the University of Michigan 2019 Doctoral Committee: Associate Professor Zaneta Nikolovska-Coleska, Co-Chair Adjunct Associate Professor Scott A. Tomlins, Co-Chair Associate Professor Eric R. Fearon Associate Professor Alexey I. Nesvizhskii Kelly R. Kennaley [email protected] ORCID iD: 0000-0003-2439-9020 © Kelly R. Kennaley 2019 Acknowledgements I have immeasurable gratitude for the unwavering support and guidance I received throughout my dissertation. First and foremost, I would like to thank my thesis advisor and mentor Dr. Scott Tomlins for entrusting me with a challenging, interesting, and impactful project. He taught me how to drive a project forward through set-backs, ask the important questions, and always consider the impact of my work. I’m truly appreciative for his commitment to ensuring that I would get the most from my graduate education. I am also grateful to the many members of the Tomlins lab that made it the supportive, collaborative, and educational environment that it was. I would like to give special thanks to those I’ve worked closely with on this project, particularly Dr. Moloy Goswami for his mentorship, Lei Lucy Wang, Dr. Sumin Han, and undergraduate students Bhavneet Singh, Travis Weiss, and Myles Barlow. I am also grateful for the support of my thesis committee, Dr. Eric Fearon, Dr. Alexey Nesvizhskii, and my co-mentor Dr. Zaneta Nikolovska-Coleska, who have offered guidance and critical evaluation since project inception. -

Analysis of Different Evolutionary Strategies to Prevent Protein Aggregation
Universitat Autònoma de Barcelona Departament de Bioquímica i Biologia Molecular and Institut de Biotecnologia i Biomedicina Analysis of Different Evolutionary Strategies to Prevent Protein Aggregation Ricardo Graña Montes Bellaterra, December 2014 Universitat Autònoma de Barcelona Departament de Bioquímica i Biologia Molecular and Institut de Biotecnologia i Biomedicina Analysis of Different Evolutionary Strategies to Prevent Protein Aggregation Doctoral thesis submitted by Ricardo Graña Montes in candidacy for the degree of Ph.D. in Biochemistry, Molecular Biology and Biomedicine from the Universitat Autònoma de Barcelona. The work described herein has been performed at the Department de Bioquímica i Biologia Molecular and at the Institut de Biotecnologia i Biomedicina, under the supervision of Prof. Salvador Ventura Zamora. Ricardo Graña Montes Prof. Salvador Ventura Zamora Bellaterra, December 2014 SUMMARY IN ENGLISH In the last 15 years, the study of protein aggregation has evolved from a mostly neglected topic of protein chemistry to a highly dynamic research area which has expanded its implications through different fields including biochemistry, biotechnology, nanotechnology and biomedicine. The analysis of protein aggregation has attracted a particular interest in the biomedical and biotechnological areas. Because, on one side, the formation of insoluble protein deposits is associated to an increasing number of human disorders, many of which present fatal pathological consequences. And on the other hand, aggregation is a frequent shortcoming in the recombinant expression of proteins at the industrial level, such as in the production of proteinaceous therapeutic agents like antibodies. Consequently, the survey of mechanisms to prevent protein aggregation is currently the focus of deep investigation with the aim to develop preventive or therapeutic methods for the intervention of these depositional disorders and to enhance the yield in the biotechnological production of proteins. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Large-Scale Analysis of Macromolecular Crowding Effects on Protein Aggregation Using a Reconstituted Cell-Free Translation System
DATA REPORT published: 08 October 2015 doi: 10.3389/fmicb.2015.01113 Large-scale analysis of macromolecular crowding effects on protein aggregation using a reconstituted cell-free translation system Tatsuya Niwa 1†, Ryota Sugimoto1† , Lisa Watanabe1, Shugo Nakamura2, Takuya Ueda3 and Hideki Taguchi1* 1 Department of Biomolecular Engineering, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama, Japan, 2 Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan, 3 Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Chiba, Japan Edited by: Proteins must fold into their native structures in the crowded cellular environment, to Salvador Ventura, perform their functions. Although such macromolecular crowding has been considered Universitat Autònoma de Barcelona, to affect the folding properties of proteins, large-scale experimental data have so far Spain Reviewed by: been lacking. Here, we individually translated 142 Escherichia coli cytoplasmic proteins Dong-Woo Lee, using a reconstituted cell-free translation system in the presence of macromolecular Kyungpook National University, South crowding reagents (MCRs), Ficoll 70 or dextran 70, and evaluated the aggregation Korea Pierre Genevaux, propensities of 142 proteins. The results showed that the MCR effects varied depending Centre National de la Recherche on the proteins, although the degree of these effects was modest. Statistical analyses Scientifique, France suggested that structural parameters were involved in the effects of the MCRs. Our *Correspondence: dataset provides a valuable resource to understand protein folding and aggregation Hideki Taguchi [email protected] inside cells. †These authors have contributed Keywords: protein aggregation, protein folding, cell-free translation system, macromolecular crowding, large- scale analysis equally to this work. -

Prion-Like Proteins in Phase Separation and Their Link to Disease
biomolecules Review Prion-Like Proteins in Phase Separation and Their Link to Disease Macy L. Sprunger and Meredith E. Jackrel * Department of Chemistry, Washington University, St. Louis, MO 63130, USA; [email protected] * Correspondence: [email protected] Abstract: Aberrant protein folding underpins many neurodegenerative diseases as well as certain myopathies and cancers. Protein misfolding can be driven by the presence of distinctive prion and prion-like regions within certain proteins. These prion and prion-like regions have also been found to drive liquid-liquid phase separation. Liquid-liquid phase separation is thought to be an important physiological process, but one that is prone to malfunction. Thus, aberrant liquid-to-solid phase transitions may drive protein aggregation and fibrillization, which could give rise to pathological inclusions. Here, we review prions and prion-like proteins, their roles in phase separation and disease, as well as potential therapeutic approaches to counter aberrant phase transitions. Keywords: prions; prion-like domains; amyloid; protein misfolding; liquid-liquid phase separation; aberrant phase transitions; chaperones 1. Introduction Protein folding is essential for life, as proteins serve structural roles, catalyze enzy- matic reactions, and transport materials through membranes and cells, among many other Citation: Sprunger, M.L.; Jackrel, functions [1]. Most globular proteins adopt a complex three-dimensional folded struc- M.E. Prion-Like Proteins in Phase ture that is well-defined and associated with specific functions. In contrast, intrinsically Separation and Their Link to Disease. disordered proteins (IDPs) do not adopt well-defined structures. Rather, IDPs occupy a Biomolecules 2021, 11, 1014. https:// highly dynamic ensemble of conformations, and these dynamic structural changes are doi.org/10.3390/biom11071014 key to the function of these proteins [2]. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

Identification of Transcriptional Mechanisms Downstream of Nf1 Gene Defeciency in Malignant Peripheral Nerve Sheath Tumors Daochun Sun Wayne State University
Wayne State University DigitalCommons@WayneState Wayne State University Dissertations 1-1-2012 Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors Daochun Sun Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Recommended Citation Sun, Daochun, "Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors" (2012). Wayne State University Dissertations. Paper 558. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. IDENTIFICATION OF TRANSCRIPTIONAL MECHANISMS DOWNSTREAM OF NF1 GENE DEFECIENCY IN MALIGNANT PERIPHERAL NERVE SHEATH TUMORS by DAOCHUN SUN DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2012 MAJOR: MOLECULAR BIOLOGY AND GENETICS Approved by: _______________________________________ Advisor Date _______________________________________ _______________________________________ _______________________________________ © COPYRIGHT BY DAOCHUN SUN 2012 All Rights Reserved DEDICATION This work is dedicated to my parents and my wife Ze Zheng for their continuous support and understanding during the years of my education. I could not achieve my goal without them. ii ACKNOWLEDGMENTS I would like to express tremendous appreciation to my mentor, Dr. Michael Tainsky. His guidance and encouragement throughout this project made this dissertation come true. I would also like to thank my committee members, Dr. Raymond Mattingly and Dr. John Reiners Jr. for their sustained attention to this project during the monthly NF1 group meetings and committee meetings, Dr. -

The HSP70 Chaperone Machinery: J Proteins As Drivers of Functional Specificity
REVIEWS The HSP70 chaperone machinery: J proteins as drivers of functional specificity Harm H. Kampinga* and Elizabeth A. Craig‡ Abstract | Heat shock 70 kDa proteins (HSP70s) are ubiquitous molecular chaperones that function in a myriad of biological processes, modulating polypeptide folding, degradation and translocation across membranes, and protein–protein interactions. This multitude of roles is not easily reconciled with the universality of the activity of HSP70s in ATP-dependent client protein-binding and release cycles. Much of the functional diversity of the HSP70s is driven by a diverse class of cofactors: J proteins. Often, multiple J proteins function with a single HSP70. Some target HSP70 activity to clients at precise locations in cells and others bind client proteins directly, thereby delivering specific clients to HSP70 and directly determining their fate. In their native cellular environment, polypeptides are participates in such diverse cellular functions. Their constantly at risk of attaining conformations that pre- functional diversity is remarkable considering that vent them from functioning properly and/or cause them within and across species, HSP70s have high sequence to aggregate into large, potentially cytotoxic complexes. identity. They share a single biochemical activity: an Molecular chaperones guide the conformation of proteins ATP-dependent client-binding and release cycle com- throughout their lifetime, preventing their aggregation bined with client protein recognition, which is typi- by protecting interactive surfaces against non-productive cally rather promiscuous. This apparent conundrum interactions. Through such inter actions, molecular chap- is resolved by the fact that HSP70s do not work alone, erones aid in the folding of nascent proteins as they are but rather as ‘HSP70 machines’, collaborating with synthesized by ribosomes, drive protein transport across and being regulated by several cofactors. -
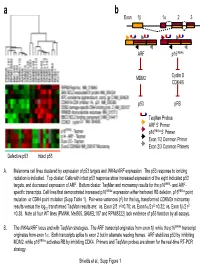
Taqman Probes ARF 5' Primer P16ink4a 5' Primer Exon 1/2
a b Exon 1β 1α 23 ARF p16INK4a MDM2 Cyclin D CDK4/6 p53 pRB TaqMan Probes ARF 5’ Primer p16INK4a 5’ Primer Exon 1/2 Common Primer Exon 2/3 Common Primers Defective p53 Intact p53 A. Melanoma cell lines clustered by expression of p53 targets and INK4a/ARF expression. The p53 response to ionizing radiation is indicated. Top cluster: Cells with intact p53 response show increased expression of the eight indicated p53 targets, and decreased expression of ARF. Bottom cluster: TaqMan and microarray results for the p16INK4- and ARF- specific transcripts. Cell lines that demonstrated increased p16INK4a expression either harbored RB deletion, p16INK4a point 2 mutation or CDK4 point mutation (Supp Table 1). Pair-wise variances (r ) for the log2 transformed CDKN2a microarray 2 2 2 results versus the log10 transformed TaqMan results are: vs. Exon 2/3 r =0.78; vs. Exon1α/2 r =0.32; vs. Exon 1β/2 r =0.38. Note all four WT lines (PMWK, Mel505, SKMEL187 and RPMI8322) lack evidence of p53 function by all assays. B. The INK4a/ARF locus and with TaqMan strategies. The ARF transcript originates from exon 1β while the p16INK4a transcript originates from exon 1α. Both transcripts splice to exon 2 but in alternate reading frames. ARF stabilizes p53 by inhibiting MDM2, while p16INK4a activates RB by inhibiting CDK4. Primers and TaqMan probes are shown for the real-time RT-PCR strategy. Shields et al., Supp Figure 1 SKMEL 28 U01 24h SKMEL WM2664 U01 48h WM2664 U01 24h 24 U01 48h SKMEL 24 U01 24h SKMEL 24 Untreated SKMEL 24 DMSO 48h SKMEL 24 DMSO 24h SKMEL WM2664 DMSO 24h WM2664 DMSO 48h WM2644 Untreated 28 Untreated SKMEL 28 DMSO 48h SKMEL 28 DMSO 24h SKMEL -3.00 -2.00 -1.00 0.00 1.00 2.00 3.00 relative to median expression Genes decreased by UO1 (863) HIF1A Hypoxia-inducible factor 1, alpha subunit NM_001530 RBBP8 Retinoblastoma binding protein 8 NM_002894 Homo sapiens, clone IMAGE:4337652, mRNA BC018676 EIF4EBP1 Eukaryotic translation initiation factor 4E binding protein EXOSC8 Exosome component 8 NM_181503 ENST00000321524 MCM7 MCM7 minichromosome maintenance deficient 7 (S. -

Prognostic and Functional Significant of Heat Shock Proteins (Hsps)
biology Article Prognostic and Functional Significant of Heat Shock Proteins (HSPs) in Breast Cancer Unveiled by Multi-Omics Approaches Miriam Buttacavoli 1,†, Gianluca Di Cara 1,†, Cesare D’Amico 1, Fabiana Geraci 1 , Ida Pucci-Minafra 2, Salvatore Feo 1 and Patrizia Cancemi 1,2,* 1 Department of Biological Chemical and Pharmaceutical Sciences and Technologies (STEBICEF), University of Palermo, 90128 Palermo, Italy; [email protected] (M.B.); [email protected] (G.D.C.); [email protected] (C.D.); [email protected] (F.G.); [email protected] (S.F.) 2 Experimental Center of Onco Biology (COBS), 90145 Palermo, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-091-2389-7330 † These authors contributed equally to this work. Simple Summary: In this study, we investigated the expression pattern and prognostic significance of the heat shock proteins (HSPs) family members in breast cancer (BC) by using several bioinfor- matics tools and proteomics investigations. Our results demonstrated that, collectively, HSPs were deregulated in BC, acting as both oncogene and onco-suppressor genes. In particular, two different HSP-clusters were significantly associated with a poor or good prognosis. Interestingly, the HSPs deregulation impacted gene expression and miRNAs regulation that, in turn, affected important bio- logical pathways involved in cell cycle, DNA replication, and receptors-mediated signaling. Finally, the proteomic identification of several HSPs members and isoforms revealed much more complexity Citation: Buttacavoli, M.; Di Cara, of HSPs roles in BC and showed that their expression is quite variable among patients. In conclusion, G.; D’Amico, C.; Geraci, F.; we elaborated two panels of HSPs that could be further explored as potential biomarkers for BC Pucci-Minafra, I.; Feo, S.; Cancemi, P.