Spatial and Temporal Wader Distribution Related to the Environment on an Intertidal Shoal in the Oosterschelde
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nordmann's Greenshank Population Analysis, at Pantai Cemara Jambi
Final Report Nordmann’s Greenshank Population Analysis, at Pantai Cemara Jambi Cipto Dwi Handono1, Ragil Siti Rihadini1, Iwan Febrianto1 and Ahmad Zulfikar Abdullah1 1Yayasan Ekologi Satwa Alam Liar Indonesia (Yayasan EKSAI/EKSAI Foundation) Surabaya, Indonesia Background Many shorebirds species have declined along East Asian-Australasian Flyway which support the highest diversity of shorebirds in the world, including the globally endangered species, Nordmann’s Greenshank. Nordmann’s Greenshank listed as endangered in the IUCN Red list of Threatened Species because of its small and declining population (BirdLife International, 2016). It’s one of the world’s most threatened shorebirds, is confined to the East Asian–Australasian Flyway (Bamford et al. 2008, BirdLife International 2001, 2012). Its global population is estimated at 500–1,000, with an estimated 100 in Malaysia, 100–200 in Thailand, 100 in Myanmar, plus unknown but low numbers in NE India, Bangladesh and Sumatra (Wetlands International 2006). The population is suspected to be rapidly decreasing due to coastal wetland development throughout Asia for industry, infrastructure and aquaculture, and the degradation of its breeding habitat in Russia by grazing Reindeer Rangifer tarandus (BirdLife International 2012). Mostly Nordmann’s Greenshanks have been recorded in very small numbers throughout Southeast Asia, and there are few places where it has been reported regularly. In Myanmar, for example, it was rediscovered after a gap of almost 129 years. The total count recorded by the Asian Waterbird Census (AWC) in 2006 for Myanmar was 28 birds with 14 being the largest number at a single locality (Naing 2007). In 2011–2012, Nordmann’s Greenshank was found three times in Sumatera Utara province, N Sumatra. -
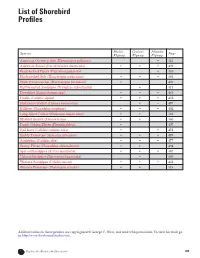
List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Flight Initiation Distances in Relation to Pedestrian and Boat Disturbance in Five Species of Waders Breeding in a Mediterranean Lagoon
Revue d’Ecologie (Terre et Vie), Vol. 73 (3), 2018: 375-384 FLIGHT INITIATION DISTANCES IN RELATION TO PEDESTRIAN AND BOAT DISTURBANCE IN FIVE SPECIES OF WADERS BREEDING IN A MEDITERRANEAN LAGOON Francesco SCARTON1 1 SELC soc. coop., Via dell’Elettricità 3/d, 30175 Marghera (Ve), Italy. E-mail: [email protected] RÉSUMÉ.— Distances d’envol de cinq espèces de limicoles nichant dans un lagon méditerranéen perturbées par des piétons ou des bateaux.— Les distances d’envol (FID) en réponse à une perturbation pédestre (N = 137) ou en bateau (N = 111) ont été mesurées chez cinq espèces d limicoles nichant dans le lagon de Venise (Italie), l’un des plus importants sites méditerranéens pour les oiseaux d’eau. Des réponses différentes au même stimulus ont été observées selon les espèces. Les différences entre les deux causes de perturbation ont été significatives chez l’Avocette qui a montré une FID plus grande à l’approche d’un bateau (T de Student = 2,18 ; ddl = 28 ; p < 0,05) alors que l’Huîtrier pie affichait une plus FID plus élevée à l’approche d’un piéton (log10 données transformées : T = -5,82 ; df = 123 ; p < 0,001). Chez l’Avocette et l’Échasse, en réponse à un piéton, la FID augmentait de manière significative avec le nombre d’oiseaux concernés par le test. Chez l’Huîtrier pie, une tendance négative significative de la FID (r = -0,47 ; p < 0,05) a été observée au cours de la saison en réponse aux perturbations par des piétons. Les distances de dissuasion, i. e. les distances aux oiseaux devant être respectées par les humains et leurs activités, ont été calculées comme FID moyenne + 2 SD, s’étalant entre 55 m (pour les bateaux vis-à-vis du Chevalier gambette) et 102 m (pour les bateaux vis-à-vis de l’Avocette). -

American Avocet Breeding Habitat, Behaviour and Use of Nesting Platforms at Kelowna, British Columbia
Avocet breeding habitat, behaviour, and nesting platform use Gyug and Weir 13 American Avocet breeding habitat, behaviour and use of nesting platforms at Kelowna, British Columbia Les W. Gyug1 and Jason T. Weir2 1 Okanagan Wildlife Consulting, 3130 Ensign Way, West Kelowna, BC V4T 1T9 [email protected] 2 Dept. of Biological Sciences and Dept. of Ecology and Evolution, University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4 [email protected] Abstract: The largest and most consistently used American Avocet (Recurvirostra americana) colony in British Columbia is located in the southern half of the former Alki Lake, Kelowna. This lake was a landfill active from the 1960’s to 1980’s, and is now slated to be filled in completely as the landfill reexpands into the remnants of the lake. Here, we report avocet behaviour, nest conditions and foraging habitat characteristics in 1999 at Alki Lake and five other wetlands in the Kelowna area to inform future mitigation strategies for this colony. Thirteen breeding pairs initiated 21 nests (including renesting after failed attempts) at Alki Lake in 1999, with no nests in other Kelowna area localities. Fifteen nests were on islands, five on 1.2 m square floating nest platforms, and one on a shoreline mudflat. Nesting on floating nest platforms had not been previously reported for American Avocets. Foraging areas regularly used by individual pairs were not necessarily adjacent to the nest, and increased from 0.32 ha during the incubation period to 0.53 ha after hatching. Avocets foraged primarily in soft silt substrates along nonvegetated shorelines and in shallow mudflats at a mean depth of 10 cm. -

08 Checklist of Species
Please note that the List below is in the sequence used in the Derbyshire Bird Report , but the monthly Bulletin BIRD NOTES will remain in the previous Voous Order pro tem . When submitting record slips, please use the EURING NUMBER shown after each species. This will greatly aid writing the Bird Notes and inputting into the computer database. The list uses British vernacular names as column 1 in the BOU BRITISH LIST A CHECKLIST of SPECIES RECORDED in DERBYSHIRE MUTE SWAN 0152 MONTAGU'S HARRIER 0263 GREAT BLACK-BACKED GULL 0600 DARTFORD WARBLER 1262 BEWICK'S SWAN 0153 GOSHAWK 0267 SOOTY TERN 0623 GRASSHOPPER WARBLER 1236 WHOOPER SWAN 0154 SPARROWHAWK 0269 LITTLE TERN 0624 SAVI'S WARBLER 1238 BEAN GOOSE 0157 BUZZARD 0287 GULL-BILLED TERN 0605 AQUATIC WARBLER 1242 PINK-FOOTED GOOSE 0158 ROUGH-LEGGED BUZZARD 0290 CASPIAN TERN 0606 SEDGE WARBLER 1243 WHITE-FRONTED GOOSE 0159 GOLDEN EAGLE 0296 WHISKERED TERN 0626 MARSH WARBLER 1250 LESSER WHITE-FRONTED GOOSE 0160 OSPREY 0301 BLACK TERN 0627 REED WARBLER 1251 GREYLAG GOOSE 0161 KESTREL 0304 WHITE-WINGED BLACK TERN 0628 GREAT REED WARBLER 1253 CANADA GOOSE 0166 RED-FOOTED FALCON 0307 SANDWICH TERN 0611 BARNACLE GOOSE 0167 MERLIN 0309 COMMON TERN 0615 WAXWING 1048 BRENT GOOSE 0168 HOBBY 0310 ROSEATE TERN 0614 NUTHATCH 1479 EGYPTIAN GOOSE 0170 PEREGRINE 0320 ARCTIC TERN 0616 TREECREEPER 1486 RUDDY SHELDUCK 0171 RAZORBILL 0636 WREN 1066 SHELDUCK 0173 WATER RAIL 0407 LITTLE AUK 0647 STARLING 1582 MANDARIN DUCK 0178 SPOTTED CRAKE 0408 PUFFIN 0654 ROSE-COLOURED STARLING 1584 WIGEON 0179 CORNCRAKE -

Status, Threats and Conservation of Birds in the German Wadden Sea
Status, threats and conservation of birds in the German Wadden Sea Technical Report Impressum – Legal notice © 2010, NABU-Bundesverband Naturschutzbund Deutschland (NABU) e.V. www.NABU.de Charitéstraße 3 D-10117 Berlin Tel. +49 (0)30.28 49 84-0 Fax +49 (0)30.28 49 84-20 00 [email protected] Text: Hermann Hötker, Stefan Schrader, Phillip Schwemmer, Nadine Oberdiek, Jan Blew Language editing: Richard Evans, Solveigh Lass-Evans Edited by: Stefan Schrader, Melanie Ossenkop Design: Christine Kuchem (www.ck-grafik-design.de) Printed by: Druckhaus Berlin-Mitte, Berlin, Germany EMAS certified, printed on 100 % recycled paper, certified environmentally friendly under the German „Blue Angel“ scheme. First edition 03/2010 Available from: NABU Natur Shop, Am Eisenwerk 13, 30519 Hannover, Germany, Tel. +49 (0)5 11.2 15 71 11, Fax +49 (0)5 11.1 23 83 14, [email protected] or at www.NABU.de/Shop Cost: 2.50 Euro per copy plus postage and packing payable by invoice. Item number 5215 Picture credits: Cover picture: M. Stock; small pictures from left to right: F. Derer, S. Schrader, M. Schäf. Status, threats and conservation of birds in the German Wadden Sea 1 Introduction .................................................................................................................................. 4 Technical Report 2 The German Wadden Sea as habitat for birds .......................................................................... 5 2.1 General description of the German Wadden Sea area .....................................................................................5 -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

CMS/CAF/Inf.4.12
CMS/CAF/Inf.4.12 Unofficial translation NATIONAL SURVEY OF TODAY SITUATION IN RUSSIAN FEDERATION WITHIN CENTRAL ASIAN FLYWAY REGION A. Brief Introduction Central-Asian Flyway covers about half a territory of Russian Federation. The main concentrations of wintering birds nesting in Russia distribute over China, India and adjusted countries. This region is considered as rather poor studied and treated as unfavourable in regards to conditions on wintering habitats, that corresponds with the growth of human activity and countries’ economical developments around the CAF. Among 176 CAF species, 143 (85 %) are located (and mostly breed) in Russian territory. The most of the species are presented by Anatidae and Wader groups. 37 species that inhabit CAF area are included in Russian Red Data Book; more then 40 species are hunting objects. B. National administrative structures for migratory waterbirds and wetlands The environment management and protection are providing by three level of power structures: federal, Subjects of Federation and municipal. Tasks for realization and regulation for maintaining of ecological laws, ecological standards and methods, environment protection, natural resources sustainable exploitation, among these, conservation and sustainable management of waterbirds’ resources are divided through different Ministries and Authorities. One of the two key Ministries is the Ministry of Natural Resources RF (MNR of Russia) which provides governmental policy at the field of sustainable exploitation, development and protection of -

EUROPEAN BIRDS of CONSERVATION CONCERN Populations, Trends and National Responsibilities
EUROPEAN BIRDS OF CONSERVATION CONCERN Populations, trends and national responsibilities COMPILED BY ANNA STANEVA AND IAN BURFIELD WITH SPONSORSHIP FROM CONTENTS Introduction 4 86 ITALY References 9 89 KOSOVO ALBANIA 10 92 LATVIA ANDORRA 14 95 LIECHTENSTEIN ARMENIA 16 97 LITHUANIA AUSTRIA 19 100 LUXEMBOURG AZERBAIJAN 22 102 MACEDONIA BELARUS 26 105 MALTA BELGIUM 29 107 MOLDOVA BOSNIA AND HERZEGOVINA 32 110 MONTENEGRO BULGARIA 35 113 NETHERLANDS CROATIA 39 116 NORWAY CYPRUS 42 119 POLAND CZECH REPUBLIC 45 122 PORTUGAL DENMARK 48 125 ROMANIA ESTONIA 51 128 RUSSIA BirdLife Europe and Central Asia is a partnership of 48 national conservation organisations and a leader in bird conservation. Our unique local to global FAROE ISLANDS DENMARK 54 132 SERBIA approach enables us to deliver high impact and long term conservation for the beneit of nature and people. BirdLife Europe and Central Asia is one of FINLAND 56 135 SLOVAKIA the six regional secretariats that compose BirdLife International. Based in Brus- sels, it supports the European and Central Asian Partnership and is present FRANCE 60 138 SLOVENIA in 47 countries including all EU Member States. With more than 4,100 staf in Europe, two million members and tens of thousands of skilled volunteers, GEORGIA 64 141 SPAIN BirdLife Europe and Central Asia, together with its national partners, owns or manages more than 6,000 nature sites totaling 320,000 hectares. GERMANY 67 145 SWEDEN GIBRALTAR UNITED KINGDOM 71 148 SWITZERLAND GREECE 72 151 TURKEY GREENLAND DENMARK 76 155 UKRAINE HUNGARY 78 159 UNITED KINGDOM ICELAND 81 162 European population sizes and trends STICHTING BIRDLIFE EUROPE GRATEFULLY ACKNOWLEDGES FINANCIAL SUPPORT FROM THE EUROPEAN COMMISSION. -

Status and Size of Pied Avocet Recurvirostra Avosetta Populations in East Africa, with a First Coastal Breeding Record
Scopus 41(2): 1–10, July 2021 Status and size of Pied Avocet Recurvirostra avosetta populations in East Africa, with a first coastal breeding record Raphaël Nussbaumer, Améline Nussbaumer, Don Turner and Colin Jackson Summary Several populations of Pied Avocet are understood to overlap in East Africa, yet the specific movements and size of each of them remains largely unclear. A review of current literature, combined with waterbird counts and recent citizen science data, suggests that potentially three populations occur in the region (Palaearctic, southern origin, and resident), and that the resident population is substantially smaller than previous estimates suggested. A new breeding record at the Kenyan coast, which only constitutes the fourth confirmed breeding location of Pied Avocet in Kenya and the first for the East African coast, demonstrates a potential overlap of Palaearctic migrants and East African residents, which may breed opportunistically along the coast. More resources are needed to carry out standardized and regular national monitoring counts in order to further elucidate the origin, movement, and numbers of Pied Avocets in East Africa. Keywords: Avocet, migration, breeding, East Africa, bird populations Introduction The Pied Avocet Recurvirostra avosetta is a monotypic wader with a global conserva- tion status of Least Concern (BirdLife International 2016), and an extensive breeding range from western Europe to Central Asia, and from the middle East to southern Africa (Pierce et al. 2020). While the origin of wintering birds in Western Africa has been researched extensively (Chambon et al. 2018, Hötker 2002) and has been con- firmed from ringing recoveries as being western Europe (Blomert et al. -

The Pied Avocet – Successful Measures Taken* DE: Säbelschnäbler DK: Klyde SE: Skärfläcka
The Pied Avocet – successful measures taken* DE: Säbelschnäbler DK: Klyde SE: Skärfläcka P-G Bentz, Lennart Karlsson, Mikael Kristersson & Göran Walinder (Falsterbo Bird Observatory, Fyren, SE-239 40 Falsterbo) The Pied Avocet Recurvirostra avosetta, an indicator species in one of the reference sites (Vellinge, SW Sweden) within EU´s LIFE-project BaltCoast, has had bad reproduction for many years at Landgren´s holme in Skanör. Extensive predation has caused heavy losses of eggs and chicks, and dramatic changes in water level have prevented the birds from breeding. In 2008 crucial measures were taken to support the breeding colony. The Pied Avocet Recurvirostra avosetta is still sea) and disturbance (people and free-running quite a rare species in Sweden and the breeding dogs). In order to protect the Pied Avocet it was population does not exceed 1500 pairs. It is decided to take some crucial measures before regarded as an endangered species due to its the breeding season 2008 started. very specific habitat demands at the breeding sites as well as in the wintering areas. According to estimates one third of the Swedish population breeds in Vellinge today. In 1994 a colony settled on the small islet Landgren´s holme in the Flommen nature reserve in the western part of the Falsterbo peninsula (Figure 1 & 2). Immediately it became a public attraction as the family life of the Pied Avocet could easily be studied from the former railway embankment in Skanör without disturbing the birds. The number of pairs has varied from year to year as well as the breeding success as described by Walinder & Karlsson (2003, 2004) and Bentz et al.