Thermophysical Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lab: Specific Heat
Lab: Specific Heat Summary Students relate thermal energy to heat capacity by comparing the heat capacities of different materials and graphing the change in temperature over time for a specific material. Students explore the idea of insulators and conductors in an attempt to apply their knowledge in real world applications, such as those that engineers would. Engineering Connection Engineers must understand the heat capacity of insulators and conductors if they wish to stay competitive in “green” enterprises. Engineers who design passive solar heating systems take advantage of the natural heat capacity characteristics of materials. In areas of cooler climates engineers chose building materials with a low heat capacity such as slate roofs in an attempt to heat the home during the day. In areas of warmer climates tile roofs are popular for their ability to store the sun's heat energy for an extended time and release it slowly after the sun sets, to prevent rapid temperature fluctuations. Engineers also consider heat capacity and thermal energy when they design food containers and household appliances. Just think about your aluminum soda can versus glass bottles! Grade Level: 9-12 Group Size: 4 Time Required: 90 minutes Activity Dependency :None Expendable Cost Per Group : US$ 5 The cost is less if thermometers are available for each group. NYS Science Standards: STANDARD 1 Analysis, Inquiry, and Design STANDARD 4 Performance indicator 2.2 Keywords: conductor, energy, energy storage, heat, specific heat capacity, insulator, thermal energy, thermometer, thermocouple Learning Objectives After this activity, students should be able to: Define heat capacity. Explain how heat capacity determines a material's ability to store thermal energy. -

Effect of Glass Transition: Density and Thermal Conductivity Measurements of B2O3
High Temperatures-High Pressures, Vol. 49, pp. 125–142 ©2020 Old City Publishing, Inc. Reprints available directly from the publisher Published by license under the OCP Science imprint, Photocopying permitted by license only a member of the Old City Publishing Group DOI: 10.32908/hthp.v49.801 Effect of glass transition: density and thermal conductivity measurements of B2O3 DMITRY CHEBYKIN1*, HANS-PETER HELLER1, IVAN SAENKO2, GERT BARTZSCH1, RIE ENDO3 AND OLENA VOLKOVA1 1Institute of Iron and Steel Technology, TU Bergakademie Freiberg, Leipziger Straße 34, 09599 Freiberg, Germany 2Institute of Materials Science, TU Bergakademie Freiberg, Gustav-Zeuner-Straße 5, 09599 Freiberg, Germany 3Department of Materials Science and Engineering, Tokyo Institute of Technology, 152–8552 Tokyo, Japan Received: May 28, 2019; Accepted: October 11, 2019. The role of B2O3 as a fluxing agent for developing fluoride free fluxes has been accentuated in the recent years. Therefore, knowledge about thermo- physical properties of the oxide are essential to find the optimal chemical composition of the mold fluxes. In the present study, the density and ther- mal conductivity of B2O3 were measured by means of the buoyancy method, the maximal bubble pressure (MBP) method and the hot-wire method in the temperature range of 295–1573 K. The results are discussed in the context of the chemical stability of the B2O3 as well as the effect of glass transition on the thermal conductivity. The density of the B2O3 decreases non-linearly with increasing temperature in the temperature range of 973–1573 K. The MBP method was successfully applied for the density measurements with a viscosity up to 91 Pa.s. -

Thermal Conductivity of Selected Materials, Part 2
. A11105 14bD4fl & TECH R,c NSfflDS-NBS 1 THT f /NSRDS-NBS * 46040 16 ' :2:196e G' 1 NBS~ piir-^ 19 . : . NBS PUBLICATIONS UNITED STATES DEPARTMENT OF COMMERCE Alexander B. Trowbridge, Secretary NATIONAL BUREAU OF STANDARDS • A. V. Astin, Director Thermal Conductivity of Selected Materials Part 2 C. Y. Ho,* R. W. Powell,*fC and P. E. Liley' *This report was prepared under contract at the Thermophysical Properties Research Center Purdue University, 2595 Yeager Road West Lafayette, Indiana 47906 NSRDS-NBS 16 National Standard Reference Data Series- National Bureau of Standards—16 (Category 5—Thermodynamic and Transport Properties) Issued February 1968 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price $2 National Bureau of Standards SEP 4 151382 QC IOO ,U573 ® n • ! (p doty X Foreword The National Standard Reference Data System is a government-wide effort to give to the technical community of the United States optimum access to the quantitative data of physical science, critically evaluated and compiled for convenience. This program was established in 1963 by the President’s Office of Science and Technology, acting upon the recommendation of the Federal Council for Science and Technology. The National Bureau of Standards has been assigned responsibility for administering the effort. The general objective of the System is to coordinate and integrate existing data evaluation and compilation activities into a systematic, comprehensive program, supplementing and expanding technical coverage when necessary, establishing and maintaining standards for the output of the participating groups, and providing mechanisms for the dissemination of the output as required. -

Thermal Properties and the Prospects of Thermal Energy Storage of Mg–25%Cu–15%Zn Eutectic Alloy As Phasechange Material
materials Article Thermal Properties and the Prospects of Thermal Energy Storage of Mg–25%Cu–15%Zn Eutectic Alloy as Phase Change Material Zheng Sun , Linfeng Li, Xiaomin Cheng *, Jiaoqun Zhu, Yuanyuan Li and Weibing Zhou School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China; [email protected] (Z.S.); [email protected] (L.L.); [email protected] (J.Z.); [email protected] (Y.L.); [email protected] (W.Z.) * Correspondence: [email protected]; Tel.: +86-13507117513 Abstract: This study focuses on the characterization of eutectic alloy, Mg–25%Cu–15%Zn with a phase change temperature of 452.6 ◦C, as a phase change material (PCM) for thermal energy storage (TES). The phase composition, microstructure, phase change temperature and enthalpy of the alloy were investigated after 100, 200, 400 and 500 thermal cycles. The results indicate that no considerable phase transformation and structural change occurred, and only a small decrease in phase transition temperature and enthalpy appeared in the alloy after 500 thermal cycles, which implied that the Mg–25%Cu–15%Zn eutectic alloy had thermal reliability with respect to repeated thermal cycling, which can provide a theoretical basis for industrial application. Thermal expansion and thermal Citation: Sun, Z.; Li, L.; Cheng, X.; conductivity of the alloy between room temperature and melting temperature were also determined. Zhu, J.; Li, Y.; Zhou, W. Thermal The thermophysical properties demonstrated that the Mg–25%Cu–15%Zn eutectic alloy can be Properties and the Prospects of considered a potential PCM for TES. -

Compression of an Ideal Gas with Temperature Dependent Specific
Session 3666 Compression of an Ideal Gas with Temperature-Dependent Specific Heat Capacities Donald W. Mueller, Jr., Hosni I. Abu-Mulaweh Engineering Department Indiana University–Purdue University Fort Wayne Fort Wayne, IN 46805-1499, USA Overview The compression of gas in a steady-state, steady-flow (SSSF) compressor is an important topic that is addressed in virtually all engineering thermodynamics courses. A typical situation encountered is when the gas inlet conditions, temperature and pressure, are known and the compressor dis- charge pressure is specified. The traditional instructional approach is to make certain assumptions about the compression process and about the gas itself. For example, a special case often consid- ered is that the compression process is reversible and adiabatic, i.e. isentropic. The gas is usually ÊÌ considered to be ideal, i.e. ÈÚ applies, with either constant or temperature-dependent spe- cific heat capacities. The constant specific heat capacity assumption allows for direct computation of the discharge temperature, while the temperature-dependent specific heat assumption does not. In this paper, a MATLAB computer model of the compression process is presented. The substance being compressed is air which is considered to be an ideal gas with temperature-dependent specific heat capacities. Two approaches are presented to describe the temperature-dependent properties of air. The first approach is to use the ideal gas table data from Ref. [1] with a look-up interpolation scheme. The second approach is to use a curve fit with the NASA Lewis coefficients [2,3]. The computer model developed makes use of a bracketing–bisection algorithm [4] to determine the temperature of the air after compression. -

R09 SI: Thermal Properties of Foods
Related Commercial Resources CHAPTER 9 THERMAL PROPERTIES OF FOODS Thermal Properties of Food Constituents ................................. 9.1 Enthalpy .................................................................................... 9.7 Thermal Properties of Foods ..................................................... 9.1 Thermal Conductivity ................................................................ 9.9 Water Content ........................................................................... 9.2 Thermal Diffusivity .................................................................. 9.17 Initial Freezing Point ................................................................. 9.2 Heat of Respiration ................................................................. 9.18 Ice Fraction ............................................................................... 9.2 Transpiration of Fresh Fruits and Vegetables ......................... 9.19 Density ...................................................................................... 9.6 Surface Heat Transfer Coefficient ........................................... 9.25 Specific Heat ............................................................................. 9.6 Symbols ................................................................................... 9.28 HERMAL properties of foods and beverages must be known rizes prediction methods for estimating these thermophysical proper- Tto perform the various heat transfer calculations involved in de- ties and includes examples on the -

THERMAL CONDUCTIVITY of CONTINUOUS CARBON FIBRE REINFORCED COPPER MATRIX COMPOSITES J.Koráb1, P.Šebo1, P.Štefánik 1, S.Kavecký 1 and G.Korb 2
1. THERMAL CONDUCTIVITY OF CONTINUOUS CARBON FIBRE REINFORCED COPPER MATRIX COMPOSITES J.Koráb1, P.Šebo1, P.Štefánik 1, S.Kavecký 1 and G.Korb 2 1 Institute of Materials and Machine Mechanics of the Slovak Academy of Sciences, Racianska 75, 836 06 Bratislava, Slovak Republic 2 Austrian Research Centre Seibersdorf, A-2444 Seibersdorf, Austria SUMMARY: The paper deals with thermal conductivity of the continuous carbon fibre reinforced - copper matrix composite that can be applied in the field of electric and electronic industry as a heat sink material. The copper matrix - carbon fibre composite with different fibre orientation and fibre content was produced by diffusion bonding of copper-coated carbon fibres. Laser flash technique was used for thermal conductivity characterisation in direction parallel and transverse to fibre orientation. The results revealed decreasing thermal conductivity as the volume content of fibre increased and independence of through thickness conductivity on fibre orientation. Achieved results were compared with the materials that are currently used as heat sinks and possibility of the copper- based composite application was analysed. KEYWORDS: thermal conductivity, copper matrix, carbon fibre, metal matrix composite, heat sink, thermal management, heat dissipation INTRODUCTION Carbon fibre reinforced - copper matrix composite (Cu-Cf MMC) offer thermomechanical properties (TMP) which are now required in electronic and electric industry. Their advantages are low density, very good thermal conductivity and tailorable coefficient of thermal expansion (CTE). These properties are important in the applications where especially thermal conductivity plays large role to solve the problems of heat dissipation due to the usage of increasingly powerful electronic components. In devices, where high density mounting technology is applied thermal management is crucial for the reliability and long life performance. -

3.37 (Class 4) Question: Thermal Diffusivity? • Thermal Diffusivity
3.37 (Class 4) Question: thermal diffusivity? • Thermal Diffusivity (alpha) = Thermal Conductivity (k) / (density * heat capacity) • Combined, or derived parameter • From Fourier’s 1st law and 2nd law (diffusion equation) • deltaH = Cp*deltaT Question: Cold welding of semiconductors, is it gold-to-gold? Typically yes, gold pad Discussion about World Trade Center and Pentagon: • Why collapse? Structural members, lose part of the group, place more stress on the others, heat from the fire softens the steel, unzipping, once they let go then they bring down the other floors by impact loading from floors above • Why no tipping? Not really any force that would create tipping. Building has a large inertia. • Defense against terrorist attacks like this? o Dealing with people who are willing to die for what they believe o Have to be willing to pay the same price, have just as strongly held beliefs as this person. o Goes back many years: Why British not able to defeat the colonials? Same about Vietnam. Afghanistan like Vietnam to Soviets. Fighting for homeland. • People have a harder time dealing with man-made disasters than natural disasters. Review: • Contamination, rough surfaces and interfacial voids limit the strength of many joints • Liquids are often used to overcome surface roughness (adhesive bonding, soldering, brazing, fusion welding) • Interfacial shear is required for mechanically produced joints • Langmuir is 10^-8 atm*sec • pressure*time product for one monolayer of atoms to strike the surface • (also need to account for sticking factor, probability that stays when hits, for oxygen and metal this will be quite high, say 0.8) Contact are under normal loading never exceeds 1/3 apparent area Story: In 1980 working with test lab (guy inherited business from father, had a correspondence degree). -

Effect of Testing Conditions on the Diffusivity Measurements of Nuclear Fuels Via the Flash Method C
Effect of testing conditions on the diffusivity measurements of nuclear fuels via the flash method C. Duguay, M. Soulon, L. Sibaud To cite this version: C. Duguay, M. Soulon, L. Sibaud. Effect of testing conditions on the diffusivity measurements of nuclear fuels via the flash method. ECerS2017 - 15th Conference and Exhibition of the European Ceramic society, Jul 2017, Budapest, Hungary. ECerS2017 - 15th Conference and Exhibition of the European Ceramic society, 2017. hal-02417740 HAL Id: hal-02417740 https://hal.archives-ouvertes.fr/hal-02417740 Submitted on 18 Dec 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Christelle DUGUAY, Mathias SOULON and Laurène SIBAUD Commissariat à l’Énergie Atomique et aux Énergies Alternatives (CEA), DEN/DEC Centre de Cadarache , 13108 Saint-Paul-Lez-Durance Cedex, France, E-mail : [email protected] Context and objectives Thermal conductivity of the nuclear fuel : λλλ ρρρ A key parameter to understand the performance of the fuel under irradiation (T) = a(T).C p(T). (T) Highly dependent on microstructure, -

Temperature & Thermal Expansion
Temperature & Thermal Expansion Temperature Zeroth Law of Thermodynamics Temperature Measurement Thermal Expansion Homework Temperature & Thermal Equilibrium Temperature – Fundamental physical quantity – Measure of average kinetic energy of molecular motion Thermal equilibrium – Two objects in thermal contact cease to have an exchange of energy The Zeroth Law of Thermodynamics If objects A and B are separately in thermal equilibrium with a third object C (the thermometer), the A and B are in thermal equilibrium with each other. Temperature Measurement In principle, any system whose physical properties change with tempera- ture can be used as a thermometer Some physical properties commonly used are – The volume of a liquid – The length of a solid – The electrical resistance of a conductor – The pressure of a gas held at constant volume – The volume of a gas held at constant pressure The Glass-Bulb Thermometer Common thermometer in everyday use Physical property that changes is the volume of a liquid - usually mercury or alcohol Since the cross-sectional area of the capillary tube is constant, the change in volume varies linearly with its length along the tube Calibrating the Thermometer The thermometer can be calibrated by putting it in thermal equilibrium with environments at known temperatures and marking the end of the liquid column Commonly used environments are – Ice-water mixture in equilibrium at the freezing point of water – Water-steam mixture in equilibrium at the boiling point of water Once the ends of the liquid column have -
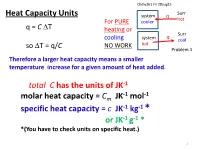
Total C Has the Units of JK-1 Molar Heat Capacity = C Specific Heat Capacity
Chmy361 Fri 28aug15 Surr Heat Capacity Units system q hot For PURE cooler q = C ∆T heating or q Surr cooling system cool ∆ C NO WORK hot so T = q/ Problem 1 Therefore a larger heat capacity means a smaller temperature increase for a given amount of heat added. total C has the units of JK-1 -1 -1 molar heat capacity = Cm JK mol specific heat capacity = c JK-1 kg-1 * or JK-1 g-1 * *(You have to check units on specific heat.) 1 Which has the higher heat capacity, water or gold? Molar Heat Cap. Specific Heat Cap. -1 -1 -1 -1 Cm Jmol K c J kg K _______________ _______________ Gold 25 129 Water 75 4184 2 From Table 2.2: 100 kg of water has a specific heat capacity of c = 4.18 kJ K-1kg-1 What will be its temperature change if 418 kJ of heat is removed? ( Relates to problem 1: hiker with wet clothes in wind loses body heat) q = C ∆T = c x mass x ∆T (assuming C is independent of temperature) ∆T = q/ (c x mass) = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = = -418 kJ / ( 4.18 kJ K-1 kg-1 x 100 kg ) = -418/418 = -1.0 K Note: Units are very important! Always attach units to each number and make sure they cancel to desired unit Problems you can do now (partially): 1b, 15, 16a,f, 19 3 A Detour into the Microscopic Realm pext Pressure of gas, p, is caused by enormous numbers of collisions on the walls of the container. -

Thermal Expansion
Protection from Protect Your Thermal Expansion Water Heater from Protection from thermal expansion is provided in a For further plumbing system by the installation of a thermal expansion tank and a temperature and information Thermal pressure relief valve (T & P Valve) at the top of the tank. contact your Expansion The thermal expansion tank controls the increased local water pressure generated within the normal operating temperature range of the water heater. The small purveyor, tank with a sealed compressible air cushion Without a functioning provides a space to store and hold the additional expanded water volume. City or County Temperature & building The T & P Valve is the primary safety feature for the water heater. The temperature portion of the Pressure Relief Valve T & P Valve is designed to open and vent water department, to the atmosphere whenever the water your water heater can temperature within the tank reaches approxi- licensed plumber º º mately 210 F (99 C). Venting allows cold water to enter the tank. or the The pressure portion of a T & P Valve is designed PNWS/AWWA to open and vent to the atmosphere whenever water pressure within the tank exceeds the Cross-Connection pressure setting on the valve. The T & P Valve is normally pre-set at 125 psi or 150 psi. Control Committee through the Water heaters installed in compliance with the current plumbing code will have the required T & P PNWS office at Valve and thermal expansion tank. For public health protection, the water purveyor may require (877) 767-2992 the installation of a check valve or backflow preventer downstream of the water meter.