Smith Mountain Lake Water Quality Monitoring Program 2020 Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of the Trophic Support Capacity of Smith Mountain Lake, Virginia, for Piscivorous Fish
ANALYSIS OF THE TROPHIC SUPPORT CAPACITY OF SMITH MOUNTAIN LAKE, VIRGINIA, FOR PISCIVOROUS FISH Michael J. Cyterski Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirement for the degree of Doctor of Philosophy in Fisheries and Wildlife Sciences John J. Ney, Chair Donald J. Orth Brian R. Murphy Clint W. Coakley James M. Berkson June, 1999 Blacksburg, Virginia Keywords: Striped Bass, Clupeids, Production, Bioenergetics, Hydroacoustics Copyright 1999, Michael J. Cyterski ANALYSIS OF THE TROPHIC SUPPORT CAPACITY OF SMITH MOUNTAIN LAKE, VIRGINIA, FOR PISCIVOROUS FISH Michael J. Cyterski (ABSTRACT) This investigation examined the adequacy of the forage base to meet current demand of piscivores in Smith Mountain Lake, Virginia. Surplus production, or the maximum sustainable supply, of alewife (Alosa pseudoharengus) and gizzard shad (Dorosoma cepedianum) were determined using data on the biomass, growth, and mortality of each species. Mean hydroacoustic alewife biomass from 1993-1998 was 37 kg/ha and mean gizzard shad cove rotenone biomass from 1990-1997 was 112 kg/ha. Mean annual alewife surplus production was determined to be 73 kg/ha and mean annual gizzard shad surplus production totaled 146 kg/ha. Bioenergetics modeling and population density estimates were utilized to derive the annual food consumption (realized demand) of the two most popular sport fish in the system, striped bass (Morone saxatilis) and largemouth bass (Micropterus salmoides). The striped bass population consumed 46 kg/ha of alewife and 27 kg/ha of gizzard shad annually. Largemouth bass ate 9 kg/ha of alewife and 15 kg/ha of gizzard shad annually. -

Archaeological Assessment of Sites 44PY7, 44PY43, 44PY152 At
ARCHAEOLOGICAL ASSESSMENT OF SITES 44PY7, 44PY43, AND 44PY152 AT LEESVILLE LAKE PITTSYLVANIA COUN1Y, VIRGINIA ~ OTHER PALEOINDIAN CLUSTERS LEESVILLE LAKE SITES Prepared for Virginia Department of Historic Resources December 1994 ~ The College Of . .• :<( WILLIAM&MARY ARCHAEOLOGICAL ASSESSMENT OF SITES 44PY7, 44PY43, AND 44PY152 AT LEESVILLE LAKE PITTSYLVANIA COUNTY, VIRGINIA Submitted to: Virginia Department of Historic Resources 221 Governor Street Richmond, Virginia 23219 Submitted by: William and Mary Center for Archaeological Research Department of Anthropology The College of William and Mary Williamsburg, Virginia 23187 Project Directors Dennis B. Blanton .)onald W. Linebaugh Authors Dennis B. Blanton William Childress Jonathan Danz Leslie Mitchell Joseph Schuldenrein Jesse Zinn December 16, 1994 ABSTRACT Sites 44PY7, 44PY43, and 44PY152 on the southern shore of Leesville Lake in Pittsylvania County were subjected to archaeological evaluation. Sites 44PY7 and 44PY152 were confirmed to contain Early ArchaicIPaleoindian horizons buried beneath 1.5 to 1.8 m of alluvium. Geoarchaeological analyses and a series of radiocarbon dates make the 44PY152 deposits among the best-documented early Holocene contexts in the region. Portions of these components have been lost to erosion, but each retains significant research potential. Site 44PY43 is a remnant of a Late Woodland village. Trenching failed to locate a palisade line, but numerous post features and possible pits were identified. This site also retains potential for recovering significant information on Late Woodland settlement in this section of the Roanoke River valley. Project results are discussed in the context of prevailing settlementlsubsistencemodels fbr the region. REPORT CONTRIBUTORS Authors: Dennis B. Blanton William Childress Jonathan Danz Leslie Mitchell Joseph Schuldenrein Jesse Zinn Graphics and Report Production Editors: Donald W. -

Final Environmental Impact Statement for Hydropower License Smith Mountain Pumped Storage Project Ferc Project No. 2210
20090807-4001 FERC PDF (Unofficial) 08/07/2009 FINAL ENVIRONMENTAL IMPACT STATEMENT Federal Energy FOR HYDROPOWER LICENSE Regulat ory Commission Office of Energy Projects FERC/FEIS-0230F August 2009 SMITH MOUNTAIN PUMPED STORAGE PROJECT FEDERAL ENERGY REGULATORY COMMISSION FERC PROJECT NO. 2210 OFFICE OF ENERGY PROJECTS V 888 FIRST STREET, NE IR WASHINGTON G INIA , DC 20426 20090807-4001 FERC PDF (Unofficial) 08/07/2009 FERC/FEIS-0230F FINAL ENVIRONMENTAL IMPACT STATEMENT FOR HYDROPOWER RELICENSING Smith Mountain Pumped Storage Project FERC Project No. 2210-169 Virginia Federal Energy Regulatory Commission Office of Energy Projects Division of Hydropower Licensing 888 First Street, NE Washington, DC 20426 August 2009 20090807-4001 FERC PDF (Unofficial) 08/07/2009 This page intentionally left blank. 20090807-4001 FERC PDF (Unofficial) 08/07/2009 FEDERAL ENERGY REGULATORY COMMISSION WASHINGTON, DC 20426 OFFICE OF ENERGY PROJECTS To the Agency or Individual Addressed: Reference: Draft Environmental Impact Statement Attached is the final environmental impact statement (EIS) for the Smith Mountain Pumped Storage Project (FERC No. 2210-169), located on the Roanoke River, within the counties of Bedford, Campbell, Franklin, and Pittsylvania, Virginia. This final EIS documents the views of governmental agencies, non-governmental organizations, affected Indian tribes, the public, the license applicant, and Commission staff. It contains staff’s evaluation of the applicant’s proposal, as well as alternatives for relicensing the Smith Mountain Project. Before the Commission makes a licensing decision, it will take into account all concerns relevant to the public interest. The final EIS will be part of the record from which the Commission will make its decision. -

Some Dam – Hydro Newstm I and Other Stuff
7/01/2011 Some Dam – Hydro NewsTM i And Other Stuff Quote of Note: “What other people think of you is none of your business.” - - Regina Brett “Good wine is a necessity of life.” - -Thomas Jefferson Ron’s wine pick of the week: Mirabile Syrah 2006 - Sicily, Italy “No nation was ever drunk when wine was cheap.” - - Thomas Jefferson Dams: (They celebrate and then move on to the next big one – removal of the 4 Snake River Dams) Rocky Barker: Dam removal movement marches to Pacific Idaho Statesman.com, 06/20/11 In September, river advocates will be holding a celebration like none seen since 1999. That was the year the Edwards Dam was removed, allowing the Kennebec River in Maine to flow free for the first time since Nathaniel Hawthorne walked its banks 160 years before. The celebration will note the removal of the Elwha and Glines dams in Washington’s Olympic peninsula. The Elwha dams are bigger and have been authorized for removal for far longer. Their removal in many ways marks the maturation of the river restoration movement that got its start when a bipartisan coalition sought to set the Kennebec River free. More than 400 dams have been removed since the Swan Falls-sized Edwards Dam came down, restoring the health of 17 miles of river. The 210-foot-high Glines Dam will be the largest to come down and that contributes to the symbolic power of the act. In October, the Condit Dam on the White Salmon River, a tributary of the Columbia, will be blasted open. -

Blackwater River Waterbodies Improved High Sediment Loadings Led to Violations of the General Standard for Aquatic Life Use in Virginia’S Blackwater River
NONPOINT SOURCE SUCCESS STORY Agricultural Best Management PracticesVirginia Improve Aquatic Life in the Blackwater River Waterbodies Improved High sediment loadings led to violations of the general standard for aquatic life use in Virginia’s Blackwater River. As a result, the Virginia Department of Environmental Quality (DEQ) added two segments of the Lower Blackwater River to the 2008 Clean Water Act (CWA) section 303(d) list of impaired waters. Landowners installed agricultural best management practices (BMPs); these decreased edge-of-field sediment loading and helped improve water quality. Because of this improvement, DEQ removed two segments of the Blackwater River from Virginia’s 2014 list of impaired waters for biological impairment. Problem The Blackwater River watershed is in Franklin County, Virginia, in the Roanoke River Basin (USGS Hydrologic Unit Code 03010101). The watershed lies north of Rocky Mount, Virginia, approximately 15 miles south of Roanoke, Virginia. The Blackwater River flows southeastward and empties into Smith Mountain Lake (Figure 1). The Upper Blackwater River watershed (70,303 acres) is 69% forest, 18% pasture and hay- land, 7% cropland and less than 1% urban. The Lower Blackwater River watershed (20,504 acres) is 58% forest, 33% agricultural, 8% urban and 2% water. Biological sampling conducted at monitoring sta- tion 4ABWR029.51 in 2004 showed Virginia Stream Condition Index (VSCI) scores of 60.7 in the spring and 50.1 in the fall. The VSCI score is a macroinver- Figure 1. The Blackwater River is in southwest Virginia. tebrate and fish community index, a composite mea- sure of the number and types of pollution-sensitive these segments was excessive sedimentation. -

Tentative Agenda
ACTION REPORT [See Agenda and Minibook for Detailed Information on Specific Items] STATE WATER CONTROL BOARD MEETING THURSDAY, OCTOBER 16, 2008 AND FRIDAY, OCTOBER 17, 2008 House Room C General Assembly Building 9th & Broad Streets Richmond, Virginia Board Members Present: W. Shelton Miles, III Komal K. Jain Thomas D. C. Walker W. Jack Kiser R. Michael McKenney Robert Wayland John B. Thompson I. Minutes (July 29, 2008) Approved Minutes II. Permits American Electric Power Smith Mountain Lake Project VWP Issued Permit – SEE PAGE 3 FOR SPECIAL CONDITIONS Cutalong VWP (Louisa County) Issued Permit III. Final Regulations Potable Water Treatment Plant VPDES General Permit Adopted Regulation Water Quality Management Plan Wasteload Allocation Amendment: New Kent County Parham Landing STP Approved Fast-Track Virginia Water Protection Permit Program Regulation – Statutory Adopted Amendments Conformity Amendments Water Quality Standards Triennial Review Adopted Amendments IV. Proposed Regulations Discharges of Storm Water Associated with Industrial Approved Proposal Activity VPDES General Permit Reissuance V. Significant Noncompliance Report Received Report VI. Consent Special Orders (VPDES Permit Program) Approved Orders Northern Regional Office Leisure Capital Corp. (Louisa Co.) Piedmont Regional Office Ennis Paint, Inc. (Henrico Co.) Richard Haywood dba Shells Unlimited (Gloucester Co.) Tidewater Regional Office Gutterman Iron & Metal Corp. (Norfolk) West Central Regional Office U.S. Army and Alliant Techsystems, Inc. (Radford) Page 1 of 6 Southwest Regional Office Dixon Lumber Co., Inc. (Wythe Co.) Valley Regional Office Town of Elkton (Rockingham Co.) City of Winchester VII. Consent Special Orders (VWP Permit Program and Others) Approved Orders Tidewater Regional Office Dismal Swamp Properties, LLC (Suffolk) City of Newport News Mr. -

Smith Mountain Lake Water Quality Monitoring Program
Smith Mountain Lake Water Quality Monitoring Program SMLA Ferrum College Prepared by Dr. Carolyn L. Thomas and Dr. David M. Johnson Life Sciences Division Ferrum College Sponsored by The Smith Mountain Lake Association Funded by American Electric Power Bedford County Franklin County Pittsylvania County Tri-county Lake Administrative Commission May 2004 SMLA WATER QUALITY MONITORING PROGRAM 2003 CONTENTS CONTENTS......................................................................................................................... i FIGURES...........................................................................................................................iii TABLES ............................................................................................................................. v 2003 Officers ....................................................................................................................vii 2003 Directors...................................................................................................................vii 2003 Smith Mountain Lake Volunteer Monitors.............................................................viii 1. EXECUTIVE SUMMARY .......................................................................................... 1 1.1 The Conclusions.......................................................................................................1 1.2 The Facts Supporting These Conclusions................................................................1 2. INTRODUCTION ....................................................................................................... -

Appendix A: Adoption Resolutions
Appendix A: Adoption Resolutions Appendix A: Adoption Resolutions Placeholder for Adoption Resolutions CVPDC Hazard Mitigation Plan 2020 Update A-1 Appendix B: FEMA Crosswalk Appendix B: FEMA Crosswalk REGULATION CHECKLIST CROSSWALK REFERENCE # ELEMENT A. PLANNING PROCESS A1. Does the Plan document the planning process, including how it was Section 3: Planning prepared and who was involved in the process for each jurisdiction? Process (Requirement §201.6(c)(1)) A2. Does the Plan document an opportunity for neighboring communities local and regional agencies involved in hazard mitigation Section 3.6: Coordination activities, agencies that have the authority to regulate development as with other Agencies, well as other interests to be involved in the planning process? Entities, and Plans (Requirement §201.6(b)(2)) A3. Does the Plan document how the public was involved in the Section 3.7: Public planning process during the drafting stage? (Requirement §201.6(b)(1)) Involvement 5.3: Planning Capabilities, A4. Does the Plan describe the review and incorporation of existing 6.0: Mitigation, 7.2: Plan plans, studies, reports, and technical information (Requirement Integration and §201.6(b)(3)) Implementation A5. Is there discussion of how the community(ies) will continue public participation in the plan maintenance process (Requirement 7.4: Public Involvement §201.6(c)(4)(iii)) A6. Is there a description of the method and schedule for keeping the 7.3: Evaluating and plan current (monitoring, evaluating and updating the mitigation plan Updating within a 5-year cycle)? (Requirement 201.6(c)(4)(i)) ELEMENT B. HAZARD IDENTIFICATION AND RISK ASSESSMENT B1. Does the Plan include a description of the type, location, and extent 4.0: Hazard Identification of all natural hazards that can affect each jurisdiction(s)? (Requirement and Risk Assessment §201.6(c)(2)(i)) B2. -

A Comparison of the Environmental Effects of Open-Loop and Closed-Loop Pumped Storage Hydropower April 2020
A Comparison of the Environmental Effects of Open-Loop and Closed-Loop Pumped Storage Hydropower April 2020 PNNL-29157 Acknowledgments This work was authored by the Pacific Northwest National Laboratory (PNNL), operated by Battelle and supported by the HydroWIRES Initiative of the U.S. Department of Energy (DOE) Water Power Technologies Office (WPTO), under award or contract number DE-AC05-76RL01830. HydroWIRES Initiative The electricity system in the United States is changing rapidly with the large-scale addition of variable renewables. The flexible capabilities of hydropower, including pumped storage hydropower (PSH), make it well-positioned to aid in integrating these variable resources while supporting grid reliability and resilience. Recognizing these challenges and opportunities, WPTO has launched a new initiative known as HydroWIRES: Water Innovation for a Resilient Electricity System.1 HydroWIRES is focused on understanding and supporting the changing role of hydropower in the evolving electricity system in the United States. Through the HydroWIRES initiative, WPTO seeks to understand and drive utilization of the full potential of hydropower resources to help reduce system-wide costs and contribute to electricity system reliability and resilience, now and into the future. HydroWIRES is distinguished in its close engagement with the DOE National Laboratories. Five National Laboratories—Argonne National Laboratory, Idaho National Laboratory, National Renewable Energy Laboratory, Oak Ridge National Laboratory, and PNNL—work as a team to provide strategic insight and develop connections across the DOE portfolio that add significant value to the HydroWIRES initiative. HydroWIRES operates in conjunction with the DOE Grid Modernization Initiative,2 which focuses on the development of new architectural concepts, tools, and technologies that measure, analyze, predict, protect, and control the grid of the future, and on enabling the institutional conditions that allow for quicker development and widespread adoption of these tools and technologies. -

Smith Mountain Lake Water Quality Monitoring Program 2018 Report
Smith Mountain Lake Water Quality Monitoring Program 2018 Report Prepared by Dr. Carolyn L. Thomas, Dr. Maria A. Puccio, Dr. Delia R. Heck, Dr. David M. Johnson, Dr. Bob R. Pohlad, Ms. Carol C. Love, School of Natural Sciences and Mathematics Ferrum College Sponsored by The Smith Mountain Lake Association Funded by American Electric Power Bedford County Regional Water Authority Smith Mountain Lake Association Virginia Department of Environmental Quality Western Virginia Water Authority (WVWA) December 17, 2018 SMLA WATER QUALITY MONITORING PROGRAM 2018 TABLE OF CONTENTS TABLE of CONTENTS .................................................................................................................... i FIGURES ........................................................................................................................................ iii TABLES .......................................................................................................................................... v 2018 Officers .................................................................................................................................. vii 2018 Directors ................................................................................................................................ vii 2018 Smith Mountain Lake Volunteer Monitors ............................................................................ viii 1. EXECUTIVE SUMMARY ........................................................................................................ -

Franklin County Health Assessment (CHNA)
Page | 1 Table of Contents Disclaimer ............................................................................................................................. 5 Acknowledgements ............................................................................................................... 6 Project Management Team ................................................................................................... 6 Community Health Assessment Team (CHAT) ........................................................................ 6 CHAT Members ................................................................................................................ 7 Executive Summary ............................................................................................................... 8 Method ............................................................................................................................ 8 Findings ........................................................................................................................... 8 Response ......................................................................................................................... 9 Target Population ................................................................................................................ 10 Service Area ........................................................................................................................ 10 Community Health Improvement Process ........................................................................... -
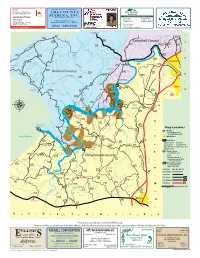
22 Mar Inside ABSOLUTE Final.Indd
State Farm® “Quality Service Year Round!” Providing Insurance and Financial Services TRI-COUNTY FIRST NATIONAL Home Office, Bloomington, Illinois 61710 MORTGAGE MARINA, INC. A DIVISION OF FIRST NATIONAL BANK OF ALTAVISTA Lynne Crittenden CPCU, Agent Between Lake Mile Marks 4 & 5 622 Broad Street Brenda M. Eades 1007-B Main Street 1261 Sunrise Loop P.O. Box 29 Assistant Vice President Altavista, VA 24517 Lynch Station, VA 24571 Altavista, VA 24517 Mortgage Loan Officer Bus 434.369.4782 Toll Free 866.369.4782 [email protected] (Phone) (434) 369-3058 (434) 369-5126 (Fax) (434) 309-7255 24 Hour Good Neighbor service® (e-mail) [email protected] To Bedford Bishops Creek 628 - 626 Carter’s Mill Road 43 Campbell County 734 Lynch Station 1 682 712 728 712 Smith Mountain Lake Parkway Goose Creek Leesville Road - Carter’s Mill Creek Clover Creek 626 732 43 2 Leesville 665 739 Altavista Carter’s Mill Road 630 924 924 Whitehouse Dundee 630 - Fairview Church Road Browntown 734 Bedford County 630 Chellis Ford Road 718 665 Tolers Ferry Road Harbor Drive 3 Old Fire Trail Road 630 638 872 Taylor Ford Road Terrapin Creek Leesville Dam 29 608 - 631 754 29 Hurt Dundee Road Clear Pointe Run Gallows Road 733 Trading Post 872 Chellis Ford Road Runaway Bay 638 4 Roach Road Terrapin Creek Bay View Rd 1 Acres Ct Hidden Cove Dalton Lane Mill Creek 638 Thomas Ct 733 unaw 665 R ay Bay N Rd - - Stoney Creek Road Stoney Creek Cliff Creek 834 Chase Run Terrapin Creek Road Stoney Creek Rd Jerimiah Dr Jacobs Hollow Heron Mount Airy Rd Rd Bay Runaway