Form and Function of the Musteloids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

APROXIMACIÓN AL LENGUAJE REPERTORIAL DE LAS ESTEREOTIPIAS EN MAMIFEROS CARNIVOROS JAGUAR (Panthera Onca), GRISÓN (Galictis
APROXIMACIÓN AL LENGUAJE REPERTORIAL DE LAS ESTEREOTIPIAS EN MAMIFEROS CARNIVOROS JAGUAR (Panthera onca), GRISÓN (Galictis vittata) Y OCELOTE (Leopardus pardalis) DEL ZOOLOGICO GUATIKA, BOYACA – TIBASOSA. Laura Inés Monsalve Zuleta CÓDIGO:2014101101 Trabajo de investigación presentado como requisito parcial para optar al título de: Médica veterinaria especialista en Medicina Homeopática veterinaria Director (a): Néstor Alberto Calderón Maldonado Médico Veterinario – Universidad de la Salle Certificado en Medicina Veterinaria Homeopática – FICH “Luis G. Páez” Especialista en Bioética – Universidad El Bosque Fundación Universitaria Escuela Colombiana de Medicina Homeopática “Luis G. Páez”. Programa de Medicina Homeopática Veterinaria Bogotá, Colombia 2016 2 DEDICATORIA A mi familia, amigos y doctores que me apoyaron en esta etapa de mi vida tanto profesional como personal. 3 AGRADECIMIENTOS Agradezco a mi familia, amigos y Doctores que me ayudaron a culminar esta etapa de mi vida. 4 TABLA DE CONTENIDO: 1. RESUMEN ...................................................................................................................................... 7 2. INTRODUCCIÓN ........................................................................................................................... 8 3. JUSTIFICACIÓN ........................................................................................................................... 9 4. ESTADO DEL ARTE ................................................................................................................. -

Predicting Greater Grison Galictis Vittata Presence from Scarce Records in the Department of Cordoba, Colombia
ORIGINAL CONTRIBUTION Predicting Greater Grison Galictis vittata presence from scarce records in the department of Cordoba, Colombia José F. GONZÁLEZ-MAYA1*, Julio J. CHACÓN PACHECO2, Javier RACERO-CASARRUBIA2, Erika HUMANEZ-LÓPEZ2 & Andrés ARIAS-ALZATE3 1. Proyecto de Conservación de Aguas y Tierras, ProCAT Colombia/Internacional, Calle 97ª # 10-62, Of. 202, Bogotá, Abstract. Colombia. The Greater Grison, Galictis vittata, is a poorly known species in Colombia. Throughout 2. Grupo de Investigación its range major knowledge gaps exist regarding its ecology and conservation. To compile Biodiversidad Unicórdoba, and analyse information about the species´ distribution records in the department of Universidad de Córdoba, Cordoba, Colombia and assess its presence probability according to landscape attributes, Montería, Córdoba, Colombia. we conducted a literature review of all wildlife studies in the region and compiled all possible direct presence records of the species in the department. We generated random 3.Grupo de Mastozoología, location points and characterized each distribution and random location by their distance to landscape attributes and land-cover type and modelled landscape presence using a Instituto de Biología, Multiple Logistic Regression approach. We found 33 records of the species in Cordoba Universidad de Antioquía. with most of the records distributed in the subregion of Alto Sinú (36%). Higher presence Medellín, Colombia. probabilities are localized in areas near forests mostly in the southern parts of the department, showing the species is still related with the largest forest blocks. Grisons Correspondence: appears to potentially tolerate some levels of disturbance but is still dependent to forest. The influence of natural habitats and abundance across the department and other areas José F. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Mammalian Predators Appropriating the Refugia of Their Prey
Mamm Res (2015) 60:285–292 DOI 10.1007/s13364-015-0236-y ORIGINAL PAPER When prey provide more than food: mammalian predators appropriating the refugia of their prey William J. Zielinski 1 Received: 30 September 2014 /Accepted: 20 July 2015 /Published online: 31 July 2015 # Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland (outside the USA) 2015 Abstract Some mammalian predators acquire both food and predators) may play disproportionately important roles in their shelter from their prey, by eating them and using the refugia communities. the prey construct. I searched the literature for examples of predators that exhibit this behavior and summarize their taxo- Keywords Predator–prey . Dens . Herbivore . Behavior . nomic affiliations, relative sizes, and distributions. I hypothe- Habitat . Resting . Foraging sized that size ratios of species involved in this dynamic would be near 1.0, and that most of these interactions would occur at intermediate and high latitudes. Seventeen species of Introduction Carnivorans exploited at least 23 species of herbivores as food and for their refugia. Most of them (76.4 %) were in the Mammals require food and most require shelter, either to pro- Mustelidae; several small species of canids and a few tect them from predators or from thermal stress. Carnivorous herpestids were exceptions. Surprisingly, the average mammals are unique in that they subsist on mobile food predator/prey weight ratio was 10.51, but few species of pred- sources which, particularly if these sources are vertebrates, ators were more than ten times the weight of the prey whose may build their own refuges to help regulate their body tem- refugia they exploit. -

Galictis Cuja Molina, 1782) As Host of Dioctophyme Renale Goeze, 1782 Furão Pequeno (Galictis Cuja Molina, 1782) Como Hospedeiro De Dioctophyme Renale Goeze, 1782
ANIMAL PARASITOLOGY / SCIENTIFIC COMMUNICATION DOI: 10.1590/1808-1657000312016 Lesser Grison (Galictis cuja Molina, 1782) as host of Dioctophyme renale Goeze, 1782 Furão Pequeno (Galictis cuja Molina, 1782) como hospedeiro de Dioctophyme renale Goeze, 1782 Daniela Pedrassani1*, Mayana Worm1, Jéssica Drechmer1, Margareth Cristina Iazzetti Santos1 ABSTRACT: The Dioctophyme renale is a helminth parasite RESUMO: O Dioctophyme renale é um helminto parasita renal of the kidney usually seen in domestic and wild carnivores and observado normalmente em carnívoros domésticos e silvestres e rarely in human beings. This is a report about the parasitism excepcionalmente em seres humanos. Relata-se o parasitismo por D. of D. renale found in the kidney of two roadkill lesser grisons renale em rim de dois furões pequenos (Galictis cuja) encontrados (Galictis cuja) in the North of the state of Santa Catarina, mortos por atropelamento no Norte do estado de Santa Catarina, Brazil. The report of this parasitism in this species is important Brasil. Relatar esse parasitismo nessa espécie é importante, para to complement the records about this native carnivore as a que se possam somar dados relativos a participação deste carnívoro contributor in the epidemiologic chain while host/disseminator nativo na cadeia epidemiológica como hospedeiro/ veiculador desse of this helminth with zoonotic potential. helminto com potencial zoonótico. KEYWORDS: Dioctophyma; wild animal; mustelids; roadkill; PALAVRAS-CHAVE: Dioctophyma; animal silvestre; mustelí- kidney parasitism. deo; atropelamento em rodovia; parasitismo renal. 1Universidade do Contestado (UnC) – Canoinhas (SC), Brazil. *Corresponding author: [email protected] Received on: 04/22/2016. Accepted on: 09/12/2017 Arq. Inst. Biol., v.84, 1-4, e0312016, 2017 1 D. -

Small Carnivores of the Mt Rungwe–Kitulo Landscape, Southwest Tanzania: Presence, Distributions and Threats
Small carnivores of the Mt Rungwe–Kitulo landscape, southwest Tanzania: presence, distributions and threats Daniela W. DE LUCA1 and Noah E. MPUNGA2 Abstract An ongoing multi-disciplinary research and conservation initiative examined the small carnivore community of the Mt Rung- we–Kitulo landscape in southwest Tanzania over an eight-year period. This key landscape’s two contiguous protected areas (Mt Rungwe Nature Reserve and Kitulo National Park) had not, at the study’s start, been formally managed for decades. We used sign survey, camera-trapping and interviews to assess small carnivore species richness and conservation status, and the causes of threat to each species, such as habitat degradation and hunting. Across the Mt Rungwe–Kitulo landscape 11 species of small car- nivores were sighted, camera-trapped and/or recorded by hunted remains and/or signs (faeces and/or footprints). Species found in Mt Rungwe were detected also in Kitulo except for Egyptian Mongoose Herpestes ichneumon, which nevertheless is likely to in- habit the latter. Faeces records from 2003 to 2010 indicated broad distributions for genet(s) Genetta (at least some, Rusty-spotted Genet G. maculata), Zorilla Ictonyx striatus and African Striped Weasel Poecilogale albinucha, Genetta being the most commonly recorded ‘species’ in the landscape. Excepting Servaline Genet G. servalina, all species expected in the landscape were found. The recorded carnivore composition showed a prevalence of generalist species, probably resulting from the degraded habitat (and are therefore at risk from isolation. Interviews demonstrated the importance of human perception and cultural values in respons- consequent invasion of the forest edge as it fragments) and a long history of hunting within the forest. -

Notes on the Distribution, Status, and Research Priorities of Little-Known Small Carnivores in Brazil
Notes on the distribution, status, and research priorities of little-known small carnivores in Brazil Tadeu G. de OLIVEIRA Abstract Ten species of small carnivores occur in Brazil, including four procyonids, four mustelids (excluding otters), and two mephitids. On the IUCN Red List of Threatened Species eight are assessed as Least Concern and two as Data Deficient. The state of knowledge of small carnivores is low compared to other carnivores: they are among the least known of all mammals in Brazil. The current delineation of Bassaricyon and Galictis congeners appears suspect and not based on credible information. Research needs include understanding dis- tributions, ecology and significant evolutionary units, with emphasis on theAmazon Weasel Mustela africana. Keywords: Amazon weasel, Data Deficient, Olingo, Crab-eating Raccoon, Hog-nosed Skunk Notas sobre la distribución, estado y prioridades de investigación de los pequeños carnívoros de Brasil Resumen En Brasil ocurren diez especies de pequeños carnívoros, incluyendo cuatro prociónidos, cuatro mustélidos (excluyendo nutrias) y dos mephitidos. De acuerdo a la Lista Roja de Especies Amenazadas de la UICN, ocho especies son evaluadas como de Baja Preocupación (LC) y dos son consideradas Deficientes de Datos (DD). El estado de conocimiento de los pequeños carnívoros es bajo comparado con otros carnívoros y se encuentran entre los mamíferos menos conocidos de Brasil. La delineación congenérica actual de Bassaricyon y Galictis parece sospechosa y no basada en información confiable. Las necesidades de investigación incluyen el entendimiento de las distribuciones, ecología y unidades evolutivas significativas, con énfasis en la ComadrejaAmazónica Mustela africana. Palabras clave: Comadreja Amazónica, Deficiente de Datos, Mapache Cangrejero, Olingo, Zorrillo Introduction 1999), but recently has been recognised (e.g. -

Musteloidea (Carnivora, Mammalia)
e390-11 Montoya.qxd 1/2/12 09:36 Página 193 Estudios Geológicos, 67(2) julio-diciembre 2011, 193-206 ISSN: 0367-0449 doi:10.3989/egeol.40576.183 Musteloidea (Carnivora, Mammalia) from the Late Miocene of Venta del Moro (Valencia, Spain) Musteloidea (Carnivora, Mammalia) del Mioceno Superior de Venta del Moro (Valencia, España) P.Montoya1, J. Morales2, J. Abella2 ABSTRACT The purpose of the present work is to describe the Musteloidea from the Late Miocene locality of Venta del Moro (Valencia, Spain). We have identified the following species: Martes ginsburgi nov. sp., Lutra affinis Gervais, 1859, Plesiogulo monspessulanus Viret, 1939 and Promephitis alexejewi Schloss- er, 1924. Besides Plesiogulo monspessulanus which was already described in this locality and in Las Casiones (MN 13, Teruel Basin), we are approaching an unedited Musteloidea assemblage from the Miocene of the Iberian Peninsula. The m1 of Martes ginsburgi nov. sp. is similar in size and morphology to the Asian species of the genus, M. anderssoni and M. zdanskyi, but it differs in having a very wide M1 with a developed lingual platform. The presence of Lutra affinis is the third register of this species in the fossil record, since it was only previously known from the Pliocene of Montpellier (France) and the termi- nal Miocene of Maramena (Greece). Promephitis alexejewi is the first appearance of this species in Europe, which has been only registered until now in several localities of Mongolia and China. It differs from the rest of Promephitis species in the possession of a narrower m1, with a very sectorial trigonid. Keywords: Mammalia, Carnivora, Musteloidea, Martes, Lutra, Plesiogulo, Promephitis, Venta del Moro, Spain, Upper Miocene RESUMEN Se describen los Musteloidea procedentes del Mioceno terminal (MN13) de Venta del Moro (Valencia, España). -

The 2008 IUCN Red Listings of the World's Small Carnivores
The 2008 IUCN red listings of the world’s small carnivores Jan SCHIPPER¹*, Michael HOFFMANN¹, J. W. DUCKWORTH² and James CONROY³ Abstract The global conservation status of all the world’s mammals was assessed for the 2008 IUCN Red List. Of the 165 species of small carni- vores recognised during the process, two are Extinct (EX), one is Critically Endangered (CR), ten are Endangered (EN), 22 Vulnerable (VU), ten Near Threatened (NT), 15 Data Deficient (DD) and 105 Least Concern. Thus, 22% of the species for which a category was assigned other than DD were assessed as threatened (i.e. CR, EN or VU), as against 25% for mammals as a whole. Among otters, seven (58%) of the 12 species for which a category was assigned were identified as threatened. This reflects their attachment to rivers and other waterbodies, and heavy trade-driven hunting. The IUCN Red List species accounts are living documents to be updated annually, and further information to refine listings is welcome. Keywords: conservation status, Critically Endangered, Data Deficient, Endangered, Extinct, global threat listing, Least Concern, Near Threatened, Vulnerable Introduction dae (skunks and stink-badgers; 12), Mustelidae (weasels, martens, otters, badgers and allies; 59), Nandiniidae (African Palm-civet The IUCN Red List of Threatened Species is the most authorita- Nandinia binotata; one), Prionodontidae ([Asian] linsangs; two), tive resource currently available on the conservation status of the Procyonidae (raccoons, coatis and allies; 14), and Viverridae (civ- world’s biodiversity. In recent years, the overall number of spe- ets, including oyans [= ‘African linsangs’]; 33). The data reported cies included on the IUCN Red List has grown rapidly, largely as on herein are freely and publicly available via the 2008 IUCN Red a result of ongoing global assessment initiatives that have helped List website (www.iucnredlist.org/mammals). -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -
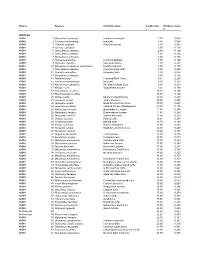
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

Exploiting Interspecific Olfactory Communication to Monitor Predators
Ecological Applications, 27(2), 2017, pp. 389–402 © 2016 by the Ecological Society of America Exploiting interspecific olfactory communication to monitor predators PATRICK M. GARVEY,1,2 ALISTAIR S. GLEN,2 MICK N. CLOUT,1 SARAH V. WYSE,1,3 MARGARET NICHOLS,4 AND ROGER P. PECH5 1Centre for Biodiversity and Biosecurity, School of Biological Sciences, University of Auckland, Auckland, New Zealand 2Landcare Research, Private Bag 92170, Auckland, 1142 New Zealand 3Royal Botanic Gardens Kew, Wakehurst Place, RH17 6TN United Kingdom 4Centre for Wildlife Management and Conservation, Lincoln University, Canterbury, New Zealand 5Landcare Research, PO Box 69040, Lincoln, 7640 New Zealand Abstract. Olfaction is the primary sense of many mammals and subordinate predators use this sense to detect dominant species, thereby reducing the risk of an encounter and facilitating coexistence. Chemical signals can act as repellents or attractants and may therefore have applications for wildlife management. We devised a field experiment to investigate whether dominant predator (ferret Mustela furo) body odor would alter the behavior of three common mesopredators: stoats (Mustela erminea), hedgehogs (Erinaceus europaeus), and ship rats (Rattus rattus). We predicted that apex predator odor would lead to increased detections, and our results support this hypothesis as predator kairomones (interspecific olfactory messages that benefit the receiver) provoked “eavesdropping” behavior by mesopredators. Stoats exhib- ited the most pronounced responses, with kairomones significantly increasing the number of observations and the time spent at a site, so that their occupancy estimates changed from rare to widespread. Behavioral responses to predator odors can therefore be exploited for conserva- tion and this avenue of research has not yet been extensively explored.