Potassium Isotopic Composition of Australasian Tektites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Terrestrial Impact Structures Provide the Only Ground Truth Against Which Computational and Experimental Results Can Be Com Pared
Ann. Rev. Earth Planet. Sci. 1987. 15:245-70 Copyright([;; /987 by Annual Reviews Inc. All rights reserved TERRESTRIAL IMI!ACT STRUCTURES ··- Richard A. F. Grieve Geophysics Division, Geological Survey of Canada, Ottawa, Ontario KIA OY3, Canada INTRODUCTION Impact structures are the dominant landform on planets that have retained portions of their earliest crust. The present surface of the Earth, however, has comparatively few recognized impact structures. This is due to its relative youthfulness and the dynamic nature of the terrestrial geosphere, both of which serve to obscure and remove the impact record. Although not generally viewed as an important terrestrial (as opposed to planetary) geologic process, the role of impact in Earth evolution is now receiving mounting consideration. For example, large-scale impact events may hav~~ been responsible for such phenomena as the formation of the Earth's moon and certain mass extinctions in the biologic record. The importance of the terrestrial impact record is greater than the relatively small number of known structures would indicate. Impact is a highly transient, high-energy event. It is inherently difficult to study through experimentation because of the problem of scale. In addition, sophisticated finite-element code calculations of impact cratering are gen erally limited to relatively early-time phenomena as a result of high com putational costs. Terrestrial impact structures provide the only ground truth against which computational and experimental results can be com pared. These structures provide information on aspects of the third dimen sion, the pre- and postimpact distribution of target lithologies, and the nature of the lithologic and mineralogic changes produced by the passage of a shock wave. -

NEW HIGH-PRECISION POTASSIUM ISOTOPES of TEKTITES. Y. Jiang1,2, H
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 1311.pdf NEW HIGH-PRECISION POTASSIUM ISOTOPES OF TEKTITES. Y. Jiang1,2, H. Chen2, B. Fegley, Jr.2, K. Lodders2, W. Hsu3, S. B. Jacobsen4, K. Wang (王昆)2. 1CAS Key Laboratory of Planetary Sciences, Purple Moun- tain Observatory, Chinese Academy of Sciences ([email protected]), 2Dept. Earth & Planetary Sciences and McDonnell Center for the Space Sciences, Washington University in St. Louis ([email protected]), 3Space Sci- ence Institute, Macau University of Science and Technology, 4Dept. Earth & Planetary Sciences, Harvard University. Introduction: Tektites are natural glassy objects crater of Hawaii (BHVO-1), are also analyzed. Hainan formed from the melting and rapid cooling of terrestri- Tektite is a typical splash-form tektite from Hainan al rocks during the high-energy impacts of meteorites, Island, China and of a dumbbell shape. We analyzed K comets, or asteroids upon the surface of the Earth [1]. isotopes of 15 chips along the longitudinal axis of its Chemical and isotopic compositions indicate that pre- profile, on a Neptune Plus MC-ICP-MS at Washington cursor components of tektites are the upper terrestrial University in St. Louis [14]. Except for Hainan tektite, continental crust, rather than extraterrestrial rocks. all other samples were only analyzed in bulk, with a Tektites are characterized by the depletion of volatile GV Instruments IsoProbe P MC-ICP-MS at Harvard elements and water, and heavy Cd, Sn, Zn and Cu iso- University following the description in [13]. K isotope topic compositions [2-5]. Since volatilities of elements compositions are reported using the per mil (‰) nota- 41 41 39 41 39 are defined by their 50% condensation temperature (Tc) tion, where δ K = ([( K/ K)sample/( K/ K)standard – of the solar nebula gas at 10-4 bars [6], there is no rea- 1]×1000). -

Chapter 5: Shock Metamorphism and Impact Melting
5. Shock Metamorphism and Impact Melting ❖❖❖ Shock-metamorphic products have become one of the diagnostic tools of impact cratering studies. They have become the main criteria used to identify structures of impact origin. They have also been used to map the distribution of shock-pressures throughout an impact target. The diverse styles of shock metamorphism include fracturing of crystals, formation of microcrystalline planes of glass through crystals, conversion of crystals to high-pressure polymorphs, conversion of crystals to glass without loss of textural integrity, conversion of crystals to melts that may or may not mix with melts from other crystals. Shock-metamorphism of target lithologies at the crater was first described by Barringer (1905, 1910) and Tilghman (1905), who recognized three different products. The first altered material they identified is rock flour, which they concluded was pulverized Coconino sandstone. Barringer observed that rock flour was composed of fragmented quartz crystals that were far smaller in size than the unaffected quartz grains in normal Coconino sandstone. Most of the pulverized silica he examined passed through a 200 mesh screen, indicating grain sizes <74 µm (0.074 mm), which is far smaller than the 0.2 mm average detrital grain size in normal Coconino (Table 2.1). Fairchild (1907) and Merrill (1908) also report a dramatic comminution of Coconino, although only 50% of Fairchild’s sample of rock flour passed through a 100 mesh screen, indicating grain sizes <149 µm. Heterogeneity of the rock flour is evident in areas where sandstone clasts survive within the rock flour. The rock flour is pervasive and a major component of the debris at the crater. -

The 3D.Y Knom Example Is /1"<
t Sixth International Congress on Glass - Washington, D. C., 1962. Fossil Glasses Produced by Inpact of Meteorites, Asteroids 2nd Possibly Comets with the Planet Xarth* A. J. Zzhen :&uon Insticute, Pittsburgh, ?ennsylvania (U. S. A. ) Sunnary / (to be trmslzted izto Frerzh and German) - i in recent thes one of the nost intriging aysteries of geGlogy has ceen the occurrence of aerodgnasicdly-shaped glasses on five continents of the earth. Tnese glasses mder discussion are obviously not of f-d- guritic origin. 3ecent research indicates that these glasses laom as tektites are the result of meteorite, esteroid, or sossibly comet hpact. Lqact glass?s, io generzl, differ Tram volcanic glasses in that they are lo;;.tr in ?,iater zontent, have laver gallium and germiun ccntents, and are rot necessarily ia mgnaticalljr unstable continental areas. These hpac- tites may be divided as follovs: (1)Glasses found in or near terrestrial neteorite craters. These glasses usually contain numerous s-,'nerules 02 nickel-iron, coesite, chunlks of partially melted meteoritic inatter and even stishovite. Shattered or fractured melted mi-nerals such as quarts are comxonly gresent. Aerodpaaic-shaping nay or nay not be present in this t-ne. &m?les are Canyon Diablo and Wabar Crster glasses. (2) Impzct- glasses zssociated with craters uitn no evidence of meteoritic mterial i? the @.ass or surrounding the explosisn site. The 3d.y knom example is /1"< Tnis vork vas supported by Xatiocal A-eroEautizs and Space P.dministretioq,$ 3esezrch Grant NsG-37-6O Supplement 1-62. Page 2 glass associated with AoueUoul Crater in the Western Sahara Desert. -

EPSC2018-833, 2018 European Planetary Science Congress 2018 Eeuropeapn Planetarsy Science Ccongress C Author(S) 2018
EPSC Abstracts Vol. 12, EPSC2018-833, 2018 European Planetary Science Congress 2018 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2018 Impact melt boulder from northern Sweden from an unknown source Timmu Kreitsmann (1), Satu Hietala (2), Tapio Soukka (3), Jüri Plado (1), Jari Nenonen (2) and Lauri J. Pesonen (4) (1) Department of Geology, University of Tartu, Estonia ([email protected], [email protected]), (2) Geological Survey of Finland, Finland ([email protected], [email protected]), (3) Oulu Mining School, University of Oulu, Finland ([email protected]) (4) Solid Earth Geophysics Laboratory, Physics Department, University of Helsinki, Finland ([email protected]) Abstract We report an impact melt rock finding from northern Sweden, near the village of Kitkiöjärvi. There is no confirmed meteorite impact structure nearby, thus, the source is currently undiscovered. The impact origin of the finding was confirmed by the presence of planar deformation features (PDFs) in quartz. 1. Introduction Impact cratering is a common and frequent process that affects the planetary surfaces across the solar system throughout geologic time. On Earth, there are 191 confirmed impact structures which are distributed unevenly around the globe. The Fennoscandian Shield houses around 10% of them. Here, we report an impact melt rock finding that originates from an unknown structure in northern Sweden. The semi-rounded impactite boulder, sized 10 × 7 cm, was found by Tapio Soukka in 2017 from a gravel pit at the western side of village Kitkiöjärvi (67°46'16"N 23°03'06"E; Fig. 1). Figure 1: Proven impact structures in Fennoscandia. -

A Tomography Approach to Investigate Impactite Structure
3rd International Conference on Tomography of Materials and Structures Lund, Sweden, 26-30 June 2017, ICTMS2017-110 A TOMOGRAPHY APPROACH TO INVESTIGATE IMPACTITE STRUCTURE A. Fedrigo*1,2, K. Marstal1,3, A. Bjorholm Dahl1, M. Lyksborg1, C. Gundlach1, M. Strobl2 & C. Bender Koch4 1Danish Technical University, Denmark 2European Spallation Source ESS ERIC, Sweden 3ERASMUS MC, Netherland 4Copenhagen University, Denmark Keywords: Neutron imaging, image registration, bimodal imaging, computed tomography. Summary: We investigate the structure of impactites from the Wabar iron meteorite impact site in Saudi Arabia. In order to understand the physical and chemical processes involved in the formation of the impactite, we applied a non-destructive investigation approach, which combines X-ray and neutron tomography. This bi-modal imaging method allowed for a better understanding of the impactite’s chemical composition. 1. INTRODUCTION Meteorite impacts have recently been recognised among the most important processes able to modify planetary surfaces. It also happens to be one of the least understood in details. In particular it is a challenge to understand both the extent of impacts and the physical and chemical changes that occur during and following the impact, where materials (e.g., rocks, sand, meteorite fragments, etc.) are subjected to temperatures of several thousands degrees and pressures of several GPa. In addition, most accessible Earth impact areas are highly affected by weathering, which causes extensive secondary chemical alterations. Iron meteorites impact on planetary surfaces cause shock-induced rock deformations that happens both at macroscopic and at microscopic level, e.g. shatter cones (macroscopic) and planar deformation features in quartz (microscopic). Another indicator often correlated to meteoritic impacts is the presence of iridium concentration anomalies [1, 2]. -
Investigating Possible Belize Tektites – Request of an Extended Database on Magnetic and Raman Spectroscopical Signature of Natural Glasses
47th Lunar and Planetary Science Conference (2016) 2482.pdf INVESTIGATING POSSIBLE BELIZE TEKTITES – REQUEST OF AN EXTENDED DATABASE ON MAGNETIC AND RAMAN SPECTROSCOPICAL SIGNATURE OF NATURAL GLASSES. V.H. Hoffmann1,2, Kaliwoda M.3, Hochleitner R.3, M. Funaki4, M. Torii5. 1Faculty of Geosciences, Dep. Geo- and Envi- ronm. Sciences, Univ. Munich; 2Dep. Geosciences, Univ. Tuebingen, 3MSCM, Munich, Germany; 4Tokyo, Japan; 5Okayama, Japan. Introduction m (gr) MS (10-9 m3/kg) Log MS Since 1992 findings of possible tektite glasses have- Belize A 1.35 123.5 2.09 been reported from Tikal region, Guatemala, and later Belize 1 0.61 131.5 2.12 from Belize, here most likely in situ. Therefore the Belize 2 0.67 128.5 2.11 existence of a Central American tektite strewn field Belize 3 0.42 178.7 2.25 was proposed [1,2]. Ages of between 780 and 820 ± Belize 4 0.33 212.8 2.33 40 ka have been determined for Belize glasses [3-5]. The radiometric age constraints are indistinguishable Belize 5 0.53 168.3 2.23 from the ages of the Australite-Indochinite tektite Belize 6 0.52 195.4 2.29 strewn field (~770 ka). However, additional investiga- Belize 7 0.36 170.5 2.23 tions on Belize glasses reported different geochemical Belize 8 0.56 129.0 2.11 signatures in comparison to the Australite-Indochinite Range 123.5 – 212.8 2.09 – 2.33 tektites [6-10]. Pantasma structure in Nicaragua was Average 159.8 2.20 proposed as a possible impact crater [11]. -

The Tennessee Meteorite Impact Sites and Changing Perspectives on Impact Cratering
UNIVERSITY OF SOUTHERN QUEENSLAND THE TENNESSEE METEORITE IMPACT SITES AND CHANGING PERSPECTIVES ON IMPACT CRATERING A dissertation submitted by Janaruth Harling Ford B.A. Cum Laude (Vanderbilt University), M. Astron. (University of Western Sydney) For the award of Doctor of Philosophy 2015 ABSTRACT Terrestrial impact structures offer astronomers and geologists opportunities to study the impact cratering process. Tennessee has four structures of interest. Information gained over the last century and a half concerning these sites is scattered throughout astronomical, geological and other specialized scientific journals, books, and literature, some of which are elusive. Gathering and compiling this widely- spread information into one historical document benefits the scientific community in general. The Wells Creek Structure is a proven impact site, and has been referred to as the ‘syntype’ cryptoexplosion structure for the United State. It was the first impact structure in the United States in which shatter cones were identified and was probably the subject of the first detailed geological report on a cryptoexplosive structure in the United States. The Wells Creek Structure displays bilateral symmetry, and three smaller ‘craters’ lie to the north of the main Wells Creek structure along its axis of symmetry. The question remains as to whether or not these structures have a common origin with the Wells Creek structure. The Flynn Creek Structure, another proven impact site, was first mentioned as a site of disturbance in Safford’s 1869 report on the geology of Tennessee. It has been noted as the terrestrial feature that bears the closest resemblance to a typical lunar crater, even though it is the probable result of a shallow marine impact. -

Geology of the Wabar Meteorite Craters, Saudi Arabia; E
Lunar and Planetary Science XXVIII 1660.PDF GEOLOGY OF THE WABAR METEORITE CRATERS, SAUDI ARABIA; E. M. Shoemaker1 and J.C. Wynn2, 1U.S. Geological Survey and Lowell Observatory, Flagstaff, AZ 86001, 2U.S. Geological Survey, Reston, VA 20192. In March of 1995, we were privileged to accompany an expedition sponsored by the Zahid Corporation of Saudi Arabia to the Wabar Craters in the Rub' Al-Khali desert (The Empty Quarter) of the southern Arabian peninsula (at 21°30.2' N latitude and 50°28.4' E longitude). Transport across the sand sheet of the northern Rub' Al-Khali was by Humvees and a 4 x 4 Volvo truck, which carried a backhoe for the purpose of exposing the structure of the crater rims. Although numerous visitors have examined the Wabar meteorite craters over the 62 years since St. John Philby's first report, no detailed investigation of the geology of the craters had been undertaken prior to our expedition. We spent 5 days during the week of March 12 at the craters carrying out a systematic survey of the craters themselves and the surrounding strewn field of impact glass and impact-formed "instant rock." Parts of three craters were exposed at the time of our visit (Fig. 1). Their diameters are estimated at 11, 64, and 116 m. We refer to these as the 11-m, Philby A, and Philby B craters. Contrary to inferences in a number of previous reports (e.g. [1], [2], [3]), the craters have been formed entirely in loose sand of the active Rub' Al- Khali sand sheet. -

HIGH PRESSURE PHASES in IMPACTITES of the ZWNSHIN CRATER/TTSSR,T1.D.Badyukov
HIGH PRESSURE PHASES IN IMPACTITES OF THE ZWNSHIN CRATER/TTSSR,T1.D.Badyukov. Vernadsky Institute of Geochemistry and Analytical Chemistry, USSR Academy of Science, Moscow, It has been suggested that most rock-forming minerals can be modified in the solid state by shock wave compression (1~2). Recent data suggest a more complicated mechanism of high pres- ure phase transitions: ~Dstallizationof the phases from im- pact melt under shock compression (3.4). In order to investiga- te the problem we looked for high density in impactites of the Zhamanshin crater, The crater was formed in a two-layer target consisting of clays, marls,quartzites, chlorite-epidote-quartz slates and so forth (5). The specimens were collected from the inside part of the crater ring bank. The high density phases were yielded from the samples of impactites by dissolution in hydrofluoric acid. Minerals were identified by optical and X-ray diffraction methods. Mineral and chemical compositions of the investigated samples are shown in table 1. According to the optical microscopy observations corundum, spinel and kyani- te could be formed from the melt but it should be noted that %hisis very difficult to establish exactly because of the sub- micron sizes of c~stallites.Tnvestigation of the shock meta- morphic quartz vein sample, composed of diaplectic quartz, dia- plectic glass and melt glass showed that stishovite i~ only in diaplectic quartz, but coesite is in diaplectic glass and some- times in melt glass in agreement with the data for the Ries crater (6). This could have been si~ggestedby the schenrtic mechanism of formation of the high pressure phases (kyanite and garnet phase). -
A High PT Carbon Impactite from Shock Coalification/Carbonization of Impact Target Vegetation K
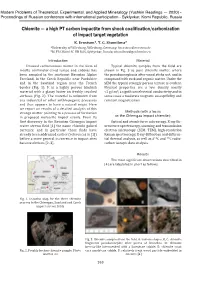
X. Минералогия астроблем и метеоритов Chiemite — a high PT carbon impactite from shock coalification/carbonization of impact target vegetation K. Ernstson1, T. G. Shumilova2 1University of Würzburg, Würzburg, Germany; [email protected] 2IG FRC Komi SC UB RAS, Syktyvkar, Russia; [email protected] Introduction Material Unusual carbonaceous matter in the form of Typical chiemite samples from the field are mostly centimetersized lumps and cobbles has shown in Fig. 3 as pure chiemite matter, where been sampled in the southeast Bavarian Alpine the pseudomorphosis after wood sticks out, and in Foreland, in the Czech Republic near Pardubice compound with rock and organic matter. Under the and in the Saarland region near the French SEM the typical strongly porous texture is evident. border (Fig. 1). It is a highly porous blackish Physical properties are a low density mostly material with a glassy luster on freshly crushed <1 g/cm3, a significant electrical conductivity and in surfaces (Fig. 2). The material is unknown from some cases a moderate magnetic susceptibility and any industrial or other anthropogenic processes remnant magnetization. and thus appears to have a natural origin. Here we report on results of a detailed analysis of this strange matter pointing to a process of formation Methods (with a focus in proposed meteorite impact events. From its on the Chiemgau impact chiemite): first discovery in the Bavarian Chiemgau impact Optical and atomic force microscopy, Xray flu crater strewn field [1] the name chiemite gained orescence spectroscopy, scanning and transmission currency, and in particular these finds have electron microscopy (SEM, TEM), highresolution already been addressed earlier (references in [1]) Raman spectroscopy, Xray diffraction and differen before a more general occurrence in impact sites tial thermal analysis, as well as δ13C and 14C radio became obvious [2, 3]. -

The Dakhleh Glass: Product of an Impact Airburst Or Cratering Event in the Western Desert of Egypt?
The Dakhleh Glass: Product of an impact airburst or cratering event in the Western Desert of Egypt? Item Type Article; text Authors Osinski, G. R.; Kieniewicz, J.; Smith, J. R.; Boslough, M. B. E.; Eccleston, M.; Schwarcz, H. P.; Kleindienst, M. R.; Haldemann, A. F. C.; Churcher, C. S. Citation Osinski, G. R., Kieniewicz, J., Smith, J. R., Boslough, M. B. E., Eccleston, M., Schwarcz, H. P., ... & Churcher, C. S. (2008). The Dakhleh Glass: Product of an impact airburst or cratering event in the Western Desert of Egypt?. Meteoritics & Planetary Science, 43(12), 2089-2107. DOI 10.1111/j.1945-5100.2008.tb00663.x Publisher The Meteoritical Society Journal Meteoritics & Planetary Science Rights Copyright © The Meteoritical Society Download date 07/10/2021 14:51:55 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final published version Link to Item http://hdl.handle.net/10150/656510 Meteoritics & Planetary Science 43, Nr 12, 2089–2107 (2008) Abstract available online at http://meteoritics.org The Dakhleh Glass: Product of an impact airburst or cratering event in the Western Desert of Egypt? Gordon R. OSINSKI1*, Johanna KIENIEWICZ2, Jennifer R. SMITH3, Mark B. E. BOSLOUGH4, Mark ECCLESTON5, Henry P. SCHWARCZ6, Maxine R. KLEINDIENST7, Albert F. C. HALDEMANN8, and Charles S. CHURCHER9 1Departments of Earth Sciences/Physics and Astronomy, University of Western Ontario, London, ON N6A 5B7, Canada 2Department of Geosciences, Denison University, Granville, Ohio 43023, USA 3Earth and Planetary Sciences, Washington University, Campus Box 1169, One Brookings Drive, Saint Louis, Missouri 63130, USA 4Sandia National Laboratories, P.O. Box 5800, Albuquerque, New Mexico 87185, USA 5Archaeology Program, La Trobe University, Bundoora 3086, Australia 6School of Geography and Earth Sciences, McMaster University, Hamilton, ON L8S 4K1, Canada 7Department of Anthropology, University of Toronto at Mississauga, 3359 Mississauga Road North, Mississauga, ON L5L 1C6, Canada 8European Space Agency, ESTEC HME-ME, P.O.