Orchid Seed Diversity Orchid Seed Diversity a Scanning Electron Microscopy Survey a Scanning Electron Microscopy Survey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORCHIDACEAE NEOTROPICALES, II During The
ORCHIDACEAE NEOTROPICALES, II De orchidaceis principaliter colombianis notutae. BY RICHARD EVANS SCHULTES During the past fifteen or twenty years, plant exp.oration in Co- lombia has been greatly intensified. The number of collections, espe- cially from the high Andean regions of the country, is now very much more comprehensive; and monographic. and floristic proj ects should find in this trend strong impetus and keen encouragement. The very task of routine identification of plant collections often uncovers sig- nificant intorrnation. Frequently, species or genera turn up which are new either to Colombia or to a given phytogeographic province of the country. Some of the exploration along frontier regions has resulted in the discovery in Colombia of species known only from neighbouring co- untries. This is especially so in the case of the orchids which have been collected in eastern or Amazonian Colombia, many of which have been reported hitherto only from Brazil or Peru. There is every reason to believe that the total number of orchids known from Co- lombia will be markedly increased as recent collections receive cri- tical attention. In 1920, Schlechter (Schlechter, R. in Repert. Sp Nov. Beih. 7 (1920) listed as known from Colombia 1293 species of orchids. The f'o'ilowing notes, chiefly on Colombian species, are based upon material preserved in the Orchid Herbarium of Oakes Ames in the Botanical Museum of Harvard University. Identification of the specimens discussed has been made by Mr. Charles Schweinfurth, Mr. Gordon de Wolf, Mr. Leslie A. Garay or the writer. The, genera. and under them the species, are arranged alphabetically. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

An Introduction to the Epiphytic Orchids of East Africa
Sphyrarchynchus sp. Cyrtorchis crassifoHa Schltr. AN INTRODUCTION TO THE EPIPHYTIC ORCHIDS OF EAST AFRICA. By W. M. MOREAU AND R. E. MOREAU. C()IYl,tents. 1. Introduction. 2. Nomenclature and classification. 3. General ecology. 4. The orchid flower. 5. Published and unpublished sources of East African records. 6. Tentative field key to the genera. 7. Annotated check-list of species. 1. INTRODUCTION. Over fifteen thousand species of orchids have been described, the vast majority of them tropical, and the greater part of them epiphytic, that is, normally growing on trees without deriving sustenance from them. But little more than ten per cent of the majestic total belong to Tropical Africa and moreover, so far as is known at present, within that area ground orchids predominate over epiphytic in the proportion of more than three to one. There is reason to believe that these figures are a reflection rather of our ignorance than of the truth. Because the Tropical African epiphytic orchids are not characterised by the magni• ficence and opulence of those of other regions, they have not attracted the commercial collector and certainly are most imperfectly known. Yet the local orchids display a delightful diversity of adaptation and of form. None are flamboyant, but many are beautiful, some are exquisitely dainty and a few are bizarre. They appeal to the same feelings and are capable of arousing the same enthusiasms as succulents or alpine plants. Moreover, anyone who takes the comparatively little trouble required to collect and grow them has the additional satisfaction of knowing that he is contributing to scientific knowledge. -

Generic and Subtribal Relationships in Neotropical Cymbidieae (Orchidaceae) Based on Matk/Ycf1 Plastid Data
LANKESTERIANA 13(3): 375—392. 2014. I N V I T E D P A P E R* GENERIC AND SUBTRIBAL RELATIONSHIPS IN NEOTROPICAL CYMBIDIEAE (ORCHIDACEAE) BASED ON MATK/YCF1 PLASTID DATA W. MARK WHITTEN1,2, KURT M. NEUBIG1 & N. H. WILLIAMS1 1Florida Museum of Natural History, University of Florida Gainesville, FL 32611-7800 USA 2Corresponding author: [email protected] ABSTRACT. Relationships among all subtribes of Neotropical Cymbidieae (Orchidaceae) were estimated using combined matK/ycf1 plastid sequence data for 289 taxa. The matrix was analyzed using RAxML. Bootstrap (BS) analyses yield 100% BS support for all subtribes except Stanhopeinae (87%). Generic relationships within subtribes are highly resolved and are generally congruent with those presented in previous studies and as summarized in Genera Orchidacearum. Relationships among subtribes are largely unresolved. The Szlachetko generic classification of Maxillariinae is not supported. A new combination is made for Maxillaria cacaoensis J.T.Atwood in Camaridium. KEY WORDS: Orchidaceae, Cymbidieae, Maxillariinae, matK, ycf1, phylogenetics, Camaridium, Maxillaria cacaoensis, Vargasiella Cymbidieae include many of the showiest align nrITS sequences across the entire tribe was Neotropical epiphytic orchids and an unparalleled unrealistic due to high levels of sequence divergence, diversity in floral rewards and pollination systems. and instead to concentrate our efforts on assembling Many researchers have posed questions such as a larger plastid data set based on two regions (matK “How many times and when has male euglossine and ycf1) that are among the most variable plastid bee pollination evolved?”(Ramírez et al. 2011), or exon regions and can be aligned with minimal “How many times have oil-reward flowers evolved?” ambiguity across broad taxonomic spans. -

Flavonoids Derivatives from Arundina Graminifolia and Their Cytotoxicity
Asian Journal of Chemistry; Vol. 25, No. 15 (2013), 8358-8360 http://dx.doi.org/10.14233/ajchem.2013.14743A Flavonoids Derivatives from Arundina graminifolia and Their Cytotoxicity 1 1,2 1 1,* 1 LIDAN SHU , YANQIONG SHEN , LIYING YANG , XUEMEI GAO and QIU-FEN HU 1Key Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Yunnan University of Nationalities, Kunming 650031, P.R. China 2Key Laboratory of Tobacco Chemistry of Yunnan Province, Yunnan Academy of Tobacco Science, Kunming 650106, P.R. China *Corresponding author: Fax: +86 871 5910017; Tel: +86 871 5910013; E-mail: [email protected] (Received: 25 October 2012; Accepted: 21 August 2013) AJC-13950 A new flavonoid, 3(S),4(S)-3',4'-dihydroxyl-7,8,-methylenedioxylpterocarpan (1), together with ten known flavonoids derivatives (2-11), were isolated from the whole plant of Arundina gramnifolia. The structure of compounds 1-11 were elucidated by spectroscopic methods including extensive 1D and 2D NMR techniques. Compound 1 was also evaluated for its cytotoxicity against five human tumor cell lines. The results revealed that compound 1 showed high cytotoxicity against HSY5Y cell with IC50 values of 2.2 µM and moderate cytotoxicities with IC50 valves 5-10 µM for other four tested cell lines. Key Words: Arundina gramnifolia, Flavonoids, Cytotoxicity. INTRODUCTION JASCO J-810 spectropolarimeter. A Tenor 27 spectrophotometer was used for scanning IR spectroscopy with KBr pellets. 1D Arundina gramnifolia (bamboo orchid) is a terrestrial plant and 2D NMR spectra were recorded on DRX-500 spectrometers belongs to species of orchid and the sole of the genus Arundina. -

Laelia5 Revista Laelia 2
Laelia REVISTA DEL GRUPO DE ESTUDIO Y CONSERVACIÓN DE ORQUÍDEAS Número 5 JULIO-AGOSTO 2009 www.gecor.org GECOR N º 5 07-08/2009 ES NOTICIA... JUNTA DIRECTIVA PROYECTO ORQUIDEA EN PÁ- sean producidas en territorio Grupo de Estudio y Conservación de orquídeas NAMA Y TAIWAN panameño. Presidente: Jose Ramón Pinela [email protected] Kevin Chen, jefe de la Misión Está propuesta es secundada Vicepresidente: Maria Jesús Arias técnica taiwanesa en Pánama por un invernadero de orquí- [email protected] afirmó recientemente que Pa- deas que ha sido creado en las Tesorería: Ana Sánchez namá junto con Taiwan colabo- instalaciones de la Universidad [email protected] ran en un proyecto para Tecnológica de Panamá con el Secretaría: Manuel Lucas [email protected] rescatar y comercializar algu- respaldo técnico de taiwan. Vocales: nos tipos de orquídeas. Diego Martínez En el Valle de Antón ubicado en [email protected] Este estudio surgió en el año el cráter de un volcán a 126 Rubén Velázquez 2008 en el distrito de Capira, km al oeste de la capital pana- [email protected] que está localizado a unos 54 meña, es un lugar dominado Emilio Esteban-Infantes km al oeste de la capital pana- por la jardinería. Existen nú- [email protected] meña, sus condiciones climáti- meros viveros en cualquier rin- cas para la agricultura son cón, rosas, orquídeas … aquí es Socios de honor Dª Gemma López Vélez semejantes a las zonas desti- donde se han seleccionado dos Dª Angela Mirro nadas a la floricultura de Tai- grupos para que reciban mate- wan. rial del laboratorio biotecnoló- gico para que se inicie el Esté proyecto científico incluye cultivo de forma organizada. -

Systematics and Evolution of the Genus Pleurothallis R. Br
Systematics and evolution of the genus Pleurothallis R. Br. (Orchidaceae) in the Greater Antilles DISSERTATION zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) im Fach Biologie eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät I der Humboldt-Universität zu Berlin von Diplom-Biologe Hagen Stenzel geb. 05.10.1967 in Berlin Präsident der Humboldt-Universität zu Berlin Prof. Dr. J. Mlynek Dekan der Mathematisch-Naturwissenschaftlichen Fakultät I Prof. Dr. M. Linscheid Gutachter/in: 1. Prof. Dr. E. Köhler 2. HD Dr. H. Dietrich 3. Prof. Dr. J. Ackerman Tag der mündlichen Prüfung: 06.02.2004 Pleurothallis obliquipetala Acuña & Schweinf. Für Jakob und Julius, die nichts unversucht ließen, um das Zustandekommen dieser Arbeit zu verhindern. Zusammenfassung Die antillanische Flora ist eine der artenreichsten der Erde. Trotz jahrhundertelanger floristischer Forschung zeigen jüngere Studien, daß der Archipel noch immer weiße Flecken beherbergt. Das trifft besonders auf die Familie der Orchideen zu, deren letzte Bearbeitung für Cuba z.B. mehr als ein halbes Jahrhundert zurückliegt. Die vorliegende Arbeit basiert auf der lang ausstehenden Revision der Orchideengattung Pleurothallis R. Br. für die Flora de Cuba. Mittels weiterer morphologischer, palynologischer, molekulargenetischer, phytogeographischer und ökologischer Untersuchungen auch eines Florenteils der anderen Großen Antillen wird die Genese der antillanischen Pleurothallis-Flora rekonstruiert. Der Archipel umfaßt mehr als 70 Arten dieser Gattung, wobei die Zahlen auf den einzelnen Inseln sehr verschieden sind: Cuba besitzt 39, Jamaica 23, Hispaniola 40 und Puerto Rico 11 Spezies. Das Zentrum der Diversität liegt im montanen Dreieck Ost-Cuba – Jamaica – Hispaniola, einer Region, die 95 % der antillanischen Arten beherbergt, wovon 75% endemisch auf einer der Inseln sind. -

Traditional Therapeutic Uses of Some Indigenous Orchids of Bangladesh
® Medicinal and Aromatic Plant Science and Biotechnology ©2009 Global Science Books Traditional Therapeutic Uses of Some Indigenous Orchids of Bangladesh Mohammad Musharof Hossain* Department of Botany, University of Chittagong, Chittagong-4331, Bangladesh Correspondence : * [email protected] ABSTRACT The traditional therapeutic uses of some indigenous orchids of Bangladesh are described in this paper. Terrestrial (11) and epiphytic (18) orchids, 29 in total, are used by Bangladeshi rural and tribal people for the treatment of nearly 45 different diseases and ailments. Roots, tubers, pseudobulbs, stems, leaves and even whole plants are used. Some herbal preparations have miraculous curative properties. Unfortunately, these preparations have not typically been subjected to the precise scientific clarification and standardization which are consequently required for clinical implementations. Some of the orchids are endangered due to over-exploitation and habitat destruction. Conservation strategies for orchids and further pharmacological studies on traditional medicines are suggested. _____________________________________________________________________________________________________________ Keywords: astavarga, conservation, ethnomedicine, herbal remedies, rasna INTRODUCTION the underground tuber of Orchis latifolia is used in the drug ‘munjatak’ pacifying cough (Khasim and Rao 1999). The Orchidaceae is the largest and most evolved family of the leaves of Vanda roxburghii have been prescribed in the flowering plants, consisting of 2500 to 35,000 species bel- ancient ‘Sanskrit’ literature for external application in rheu- onging to 750-800 genera (Dressler 1993). They are found matism, ear infections, fractures and diseases of nervous in virtually all regions around the world except the icy system. Besides these, other orchids used in local systems Antarctica, but their greatest diversity occurs in tropical and of medicine are Cleisostoma williamsonii (for bone frac- sub-tropical regions. -

Aphyllorchis Queenslandica Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Aphyllorchis queenslandica Click on images to enlarge Family Orchidaceae Scientific Name Aphyllorchis queenslandica Dockrill Dockrill, A.W. (1965) Orchadian 1: 115. Type: Queensland, Helenvale, May 1962, C. Le Roy: Holo: QRS. Common name Herbarium specimen. Copyright CSIRO Yellow Pauper Orchid Stem Above ground part of the plant (peduncle + inflorescence) about 65-90 cm tall. Leaves Plant devoid of chlorophyll. 'Leaves' (bracts) about 8-10 per plant, sessile, about 0.5-3 x 0.6-1.1 cm. Bracts 3-veined, venation longitudinal and parallel. Flowers Sepals about 13 x 2.5 mm. Petals about 11 x 2 mm, labellum larger. Labellum cream, margins upturned, purple. Column purple at the base, but yellow towards the apex. Stamen fused to the style to form a column. Staminal column about 7 x 1.5 mm. Ovary about 12 x 2 mm, outer surface with 6 longitudinal ribs. Fruit Inflorescence about 40-70 cm long, usually 6-12-flowered. Peduncle with up to 10 stem-clasping bracts, each bract about 5-20 x 6-10 mm. Bracts subtending the flowers about 10 x 2 mm and are not stem-clasping. Flowers about 20 mm wide on pedicels about 2-3 mm long. Sepals about 11-13 c 3-4 mm. Petals about 11 x 3 mm. Labellum about 6 x 2.5 mm. Column about 6-8 x 1.5 mm, curved, semi-cylindrical. -
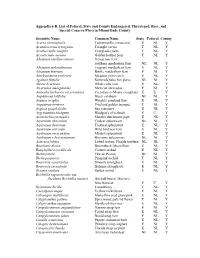
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca. -

Orchid Historical Biogeography, Diversification, Antarctica and The
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Orchid historical biogeography, ARTICLE diversification, Antarctica and the paradox of orchid dispersal Thomas J. Givnish1*, Daniel Spalink1, Mercedes Ames1, Stephanie P. Lyon1, Steven J. Hunter1, Alejandro Zuluaga1,2, Alfonso Doucette1, Giovanny Giraldo Caro1, James McDaniel1, Mark A. Clements3, Mary T. K. Arroyo4, Lorena Endara5, Ricardo Kriebel1, Norris H. Williams5 and Kenneth M. Cameron1 1Department of Botany, University of ABSTRACT Wisconsin-Madison, Madison, WI 53706, Aim Orchidaceae is the most species-rich angiosperm family and has one of USA, 2Departamento de Biologıa, the broadest distributions. Until now, the lack of a well-resolved phylogeny has Universidad del Valle, Cali, Colombia, 3Centre for Australian National Biodiversity prevented analyses of orchid historical biogeography. In this study, we use such Research, Canberra, ACT 2601, Australia, a phylogeny to estimate the geographical spread of orchids, evaluate the impor- 4Institute of Ecology and Biodiversity, tance of different regions in their diversification and assess the role of long-dis- Facultad de Ciencias, Universidad de Chile, tance dispersal (LDD) in generating orchid diversity. 5 Santiago, Chile, Department of Biology, Location Global. University of Florida, Gainesville, FL 32611, USA Methods Analyses use a phylogeny including species representing all five orchid subfamilies and almost all tribes and subtribes, calibrated against 17 angiosperm fossils. We estimated historical biogeography and assessed the