Nota Lepidopterologica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Lepidoptera Rapa Island
J. F. GATES CLA, The Lepidoptera Rapa Island SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1971 NUMBER 56 .-24 f O si % r 17401 •% -390O i 112100) 0 is -•^ i BLAKE*w 1PLATEALP I5 i I >k =(M&2l2Jo SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 56 j. F. Gates Clarke The Lepidoptera of Rapa Island SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1971 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Insti- tution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -

DD34E DNA Transposable Elements of Mosquitoes: Whole- Genome Survey, Evolution, and Transposition
DD34E DNA Transposable Elements of Mosquitoes: Whole- genome survey, evolution, and transposition Monique R. Coy Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Biochemistry Zhijian (Jake) Tu, Chair Zachary Adelman Glenda Gillaspy Peter J Kennelly John McDowell (13th of June 2007) Blacksburg, Virginia Keywords: DNA transposable element, evolution, horizontal transfer, gambol, interspersed repeat, mosquito, Tc1, transposition assay i DD34E DNA Transposable Elements of Mosquitoes: Whole-genome survey, evolution, and transposition Monique Royer Coy Abstract Transposable elements (TEs) are mobile genetic elements capable of replicating and spreading within, and in some cases, between genomes. I describe a whole-genome analysis of DD34E TEs, which belong to the IS630-Tc1-mariner superfamily of DNA transposable elements, in the African malaria mosquito, Anopheles gambiae. Twenty-six new transposons as well as a new family, gambol, were identified. The gambol family shares the DD34E catalytic motif with Tc1-DD34E transposons, but is distinct from these elements in their phylogenetic relationships. Although gambol appears to be related to a few DD34E transposons from cyanobacteria and fungi, no gambol elements have been reported in any other insects or animals thus far. This discovery expands the already expansive diversity of the IS630-Tc1-mariner TEs, and raises interesting questions as to the origin of gambol elements and their apparent diversity in An. gambiae. Several DD34E transposons discovered in An. gambiae possess characteristics that are associated with recent transposition, such as high sequence identity between copies, and intact terminal-inverted repeats and open reading frames. -

National Biodiversity Strategy and Action Plan of Georgia
Biodiversity Strategy and Action Plan - Georgia – Tbilisi, 2005 Foreword Georgia signed the Convention on Biological Diversity in 1994, thus accepting responsibility to safeguard the nation’s rich diversity of plant, animal, and microbial life, to begin using biological resources in sustainable way, and to ensure equitable sharing of benefits from biodiversity. Later the country joined other conventions including the Convention on Climate Change, the Ramsar Convention on Wetlands, CITES and the Bonn Convention. As a signatory to these important international environmental treaties, Georgia enters the world scene with the potential for joining the most advanced nations in the field of environmental protection. At the present moment of transition, Georgia has a unique opportunity to use the early experiences of other countries, and avoid irreversible changes in the quality of its environment. The national legislation on environmental protection adopted over the past few years provides an adequate legal basis for this, although further elaboration and reinforcement of the existing legislation is needed. With the Ministry of Environment being currently reorganised and assuming broader responsibilities, Georgia’s institutional arrangements for environmental protection already has the necessary structure for improving the quality of the environment throughout the country. The role of non-governmental groups has been very important in resolving problems related to nature conservation. Georgia has shown an excellent example of co-operation between governmental and non-governmental organizations in the field of environment, and particularly in the field of biodiversity conservation. After signing the Convention on Biological Diversity, the Georgian Government immediately acted to develop a Biodiversity Country Study, in partnership with UNEP, and implemented by NACRES, a local conservation organisation. -

Pala Earctic G Rassland S
Issue 46 (July 2020) ISSN 2627-9827 - DOI 10.21570/EDGG.PG.46 Journal of the Eurasian Dry Grassland Group Dry Grassland of the Eurasian Journal PALAEARCTIC GRASSLANDS PALAEARCTIC 2 Palaearctic Grasslands 46 ( J u ly 20 2 0) Table of Contents Palaearctic Grasslands ISSN 2627-9827 DOI 10.21570/EDGG.PG46 Palaearctic Grasslands, formerly published under the names Bulletin of the European Editorial 3 Dry Grassland Group (Issues 1-26) and Bulletin of the Eurasian Dry Grassland Group (Issues 27-36) is the journal of the Eurasian Dry Grassland Group (EDGG). It usually appears in four issues per year. Palaearctic Grasslands publishes news and announce- ments of EDGG, its projects, related organisations and its members. At the same time it serves as outlet for scientific articles and photo contributions. News 4 Palaearctic Grasslands is sent to all EDGG members and, together with all previous issues, it is also freely available at http://edgg.org/publications/bulletin. All content (text, photos, figures) in Palaearctic Grasslands is open access and available under the Creative Commons license CC-BY-SA 4.0 that allow to re-use it provided EDGG Publications 8 proper attribution is made to the originators ("BY") and the new item is licensed in the same way ("SA" = "share alike"). Scientific articles (Research Articles, Reviews, Forum Articles, Scientific Reports) should be submitted to Jürgen Dengler ([email protected]), following the Au- Aleksanyan et al.: Biodiversity of 12 thor Guidelines updated in Palaearctic Grasslands 45: 4. They are subject to editorial dry grasslands in Armenia: First review, with one member of the Editorial Board serving as Scientific Editor and deciding results from the 13th EDGG Field about acceptance, necessary revisions or rejection. -

Recovery of Spider Communities After a Spontaneous Summer Fire in the Forb-Bunchgrass Steppe of Eastern Ukraine
HACQUETIA 14/1 • 2015, 79–96 DOI: 10.1515/hacq-2015-0015 RECOVERY OF SPIDER COMMUNITIES AFTER A SPONTANEOUS SUMMER FIRE IN THE FORB-BUNCHGRASS STEPPE OF EASTERN UKRAINE Nina POLCHANINOVA* Abstract Recently, anthropogenic fires in protected areas have become more frequent. I studied the response of the spider community after an extensive summer fire in the ‘Striltsivskyi Steppe’ Reserve in eastern Ukraine. A total of 117 spider species was found in the pre-fire period, 40 species were registered in the first and 89 species in the third post-fire year. Herb-dwelling spiders began to colonize burnt plots in July, when juveniles of the new generation began dispersing. In September, their abundance was similar to that of undisturbed steppe and within three years, the spider assemblages recovered almost completely. Cursorial ground-dwellers in the first post-fire year decreased in species richness and increased in activity density. In the third year, their species diversity and activity density became much higher than in control plots. Some xerophilous species benefited from the fire. Litter dwellers are extremely vulnerable. During the three post-fire years, their numbers and diversity did not recover. Some rare species with a patchy geographical distribution disappeared from the local fauna. Such a reaction of endangered species is the main restriction on the use of fire as a conservation management. The prerequisite for its implementation is maintaining relevant refuges for threatened species. Keywords: endangered species, post-fire recovery, spider assemblage, ‘Striltsivskyi Steppe’ Reserve, summer fire. Izvleček Antropogeni požari na zavarovanih območjih so v zadnjem času vse bolj pogosti. -
Научный Журнал Issn 2414-4738
Научный журнал ISSN 2414-4738 МИНИСТЕРСТВО ОБРАЗОВАНИЯ И НАУКИ РОССИЙСКОЙ ФЕДЕРАЦИИ КРЫМСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ ИМЕНИ В. И. ВЕРНАДСКОГО ЭКОСИСТЕМЫ ВЫПУСК 5 (35) 2016 НАУЧНЫЙ ЖУРНАЛ ● ОСНОВАН В 1979 ГОДУ ● ВЫХОДИТ 4 РАЗА В ГОД ● СИМФЕРОПОЛЬ Свидетельство о регистрации СМИ – ПИ №ФС77-61820 от 18 мая 2015 г. ISSN 2414-4738 Печатается по решению Ученого совета Крымского федерального университета имени В. И. Вернадского, протокол № 10 от 27 октября 2016 г. В журнале публикуются материалы комплексных исследований по изучению флоры, фауны, фито- и зооценологии, экологии и биологии видов, охране растительного и животного мира. Редакционный совет журнала Главный редактор Чуян Е. Н., д. б. н., профессор Редакторы Иванов С. П., д. б. н., профессор Котов С. Ф., к. б. н., доцент Технический редактор Сволынский А. Д. Ответственный секретарь Николенко В. В., к. б. н. Члены редакционного совета Ена А. В., д. б. н., профессор Корженевский В. В., д. б. н., профессор Ермаков Н. Б., д. б. н. Маслов И. И., д. б. н. Захаренко Г. С., д. б. н., профессор Оберемок В. В., к. б. н., доцент Ивашов А. В., д. б. н., профессор Репецкая А. И., к. б. н., доцент Коба В. П., д. б. н., профессор Симагина Н. О., к. б. н., доцент Коношенко С. В. д. б. н., профессор Темурьянц Н. А., д. б. н., профессор Коренюк И. И., д. б. н., профессор Адрес редакции: Крымский федеральный университет имени В. И. Вернадского, кафедра ботаники и физиологии растений и биотехнологии, пр. Академика Вернадского, 4, Симферополь, 295007 E-mail: [email protected] Полнотекстовые версии статей последних выпусков журнала в формате PDF и правила для авторов размещены на официальном сайте журнала по адресу http://science.cfuv.ru/nauchnye-zhurnaly- kfu/ekosistemy Оригинал-макет: А. -

Recovery of Spider Communities After a Spontaneous Summer Fire in the Forb-Bunchgrass Steppe of Eastern Ukraine
HACQUETIA 14/1 • 2015, 79–96 Doi: 10.1515/hacq-2015-0015 RECOVERY OF SPIDER COMMUNItIES AFTER A SPONTANEOUS SUMMER fIRE In THE FORB-BUNCHGRASS STEPPE OF eASTERN uKrAINE nina PoLChaninoVa* Abstract recently, anthropogenic fires in protected areas have become more frequent. i studied the response of the spider community after an extensive summer fire in the ‘striltsivskyi steppe’ reserve in eastern ukraine. a total of 117 spider species was found in the pre-fire period, 40 species were registered in the first and 89 species in the third post-fire year. herb-dwelling spiders began to colonize burnt plots in July, when juveniles of the new generation began dispersing. in september, their abundance was similar to that of undisturbed steppe and within three years, the spider assemblages recovered almost completely. Cursorial ground-dwellers in the first post-fire year decreased in species richness and increased in activity density. in the third year, their species diversity and activity density became much higher than in control plots. some xerophilous species benefited from the fire. Litter dwellers are extremely vulnerable. During the three post-fire years, their numbers and diversity did not recover. some rare species with a patchy geographical distribution disappeared from the local fauna. such a reaction of endangered species is the main restriction on the use of fire as a conservation management. the prerequisite for its implementation is maintaining relevant refuges for threatened species. Keywords: endangered species, post-fire recovery, spider assemblage, ‘striltsivskyi steppe’ reserve, summer fire. Izvleček antropogeni požari na zavarovanih območjih so v zadnjem času vse bolj pogosti. -

A Melitaea Ornata (Lepidoptera: Nymphalidae) Taxonómiája, Elterjedése És Ökológiája
1949 Taxonomy, distribution and ecology of Melitaea ornata (Lepidoptera: Nymphalidae) A Melitaea ornata (Lepidoptera: Nymphalidae) taxonómiája, elterjedése és ökológiája Egyetemi doktori (PhD) értekezés Tóth János Pál Témavezető: Dr. Varga Zoltán DEBRECENI EGYETEM Természettudományi Doktori Tanács Juhász-Nagy Pál Doktori Iskola Debrecen, 2012 Ezen értekezést a Debreceni Egyetem Természettudományi Doktori Tanács Juhász- Nagy Pál Doktori Iskola Biodiverzitás programja keretében készítettem a Debreceni Egyetem természettudományi doktori (PhD) fokozatának elnyerése céljából. Tóth János Pál Debrecen, 2012. 07. 04. Tanúsítom, hogy Tóth János Pál doktorjelölt 2006-2009. között a fent megnevezett Doktori Iskola Biodiverzitás programjának keretében irányításommal végezte munkáját. Az értekezésben foglalt eredményekhez a jelölt önálló alkotó tevékenységével meghatározóan hozzájárult. Az értekezés elfogadását javasolom. Dr. Varga Zoltán Debrecen, 2012. 07. 04. 2 Taxonomy, distribution and ecology of Melitaea ornata (Lepidoptera: Nymphalidae) A Melitaea ornata (Lepidoptera: Nymphalidae) taxonómiája, elterjedése és ökológiája Értekezés a doktori (Ph.D.) fokozat megszerzése érdekében a biológia tudományágban Írta: Tóth János Pál okleveles biológus Készült a Debreceni Egyetem Juhász-Nagy Pál Doktori Iskolája (Biodiverzitás programja) keretében Témavezető: Dr. Varga Zoltán ………………………… A doktori szigorlati bizottság: elnök: ………………………… ………………………… tagok: ………………………… ………………………… ………………………… ………………………… A doktori szigorlat időpontja: ………………………… Az értekezés bírálói: -
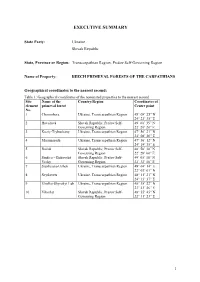
Nomination File 1133
EXECUTIVE SUMMARY State Party: Ukraine Slovak Republic State, Province or Region: Transcarpathian Region, Prešov Self-Governing Region Name of Property: BEECH PRIMEVAL FORESTS OF THE CARPATHIANS Geographical coordinates to the nearest second: Table 1: Geographical coordinates of the nominated properties to the nearest second Site Name of the Country/Region Coordinates of element primeval forest Centre point No. 1 Chornohora Ukraine, Transcarpathian Region 48° 08’ 25” N 24° 23’ 35” E 2 Havešová Slovak Republic, Prešov Self- 49˚ 00’ 35” N Governing Region 22˚ 20’ 20” E 3 Kuziy-Trybushany Ukraine, Transcarpathian Region 47° 56’ 21” N 24° 08’ 26” E 4 Maramarosh Ukraine, Transcarpathian Region 47° 56’ 12” N 24° 19’ 35” E 5 Rožok Slovak Republic, Prešov Self- 48˚ 58’ 30” N Governing Region 22˚ 28’ 00” E 6 Stužica – Bukovské Slovak Republic, Prešov Self- 49˚ 05’ 10” N Vrchy Governing Region 22˚ 32’ 10” E 7 Stuzhytsia-Uzhok Ukraine, Transcarpathian Region 49° 04’ 14” E 22° 03’ 01” N 8 Svydovets Ukraine, Transcarpathian Region 48° 11’ 21” N 24° 13’ 37” E 9 Uholka-Shyrokyi Luh Ukraine, Transcarpathian Region 48° 18’ 22” N 23° 41’ 46” E 10 Vihorlat Slovak Republic, Prešov Self- 48° 55’ 45” N Governing Region 22° 11’ 23” E 1 Textual description of the boundaries of the nominated properties: General outline of the serial nominated property The principal axis of the serial transnational nominated property “Beech primeval forests of the Carpathians” is approximately 185 km long. It coincides with the divison between the sub-provinces of Outer Eastern Carpathians and the Inner Eastern Carpathians, extending from Maramorosh on the northern megaslope of the Rakhiv Mountains and the southern macroslope of the Chornohirskyi Range in the South-East, along the Polonynian Ridge (Polonyns'kyi chrebet) up to the Bukovské Vrchy Mts. -

The First Record of Diacrisia Metelkana (Lederer, 1861) for Kazakhstan with Notes on Its Bionomics and Distribution (Lepidoptera, Erebidae, Arctiinae, Arctiini)
Ecologica Montenegrina 26: 96-101 (2019) This journal is available online at: www.biotaxa.org/em The first record of Diacrisia metelkana (Lederer, 1861) for Kazakhstan with notes on its bionomics and distribution (Lepidoptera, Erebidae, Arctiinae, Arctiini) SERGEY V. TITOV1 & ANTON V. VOLYNKIN2, 3* 1 Department of Biology and Ecology; the Research Centre for Environmental "Monitoring", Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan 2Altai State University, Lenina Avenue, 61, RF-656049, Barnaul, Russia 3 National Research Tomsk State University, Lenina Avenue, 36, RF-634050, Tomsk, Russia * Corresponding author. E-mail: [email protected] Received 29 October 2019 │ Accepted by V. Pešić: 12 December 2019 │ Published online 15 December 2019. Abstract The Amphipalaearctic tiger moth species Diacrisia metelkana (Lederer, 1861) is reported for Kazakhstan for the first time. Data on species’ bionomics in Kazakhstan and general distribution are provided. Species’ habitats in Kazakhstan are illustrated. Key words: distribution, new record, biogeography, geomorphology, bionomics, Pavlodar Region. Introduction To this day, there is no paper generalizing data on the Arctiini fauna of Kazakhstan. Nevertheless, data on Kazakhstan tiger moths can be found in some separated publications devoted to regional or local faunas and taxonomy of various groups (Dubatolov 1996; Dubatolov & de Vos 2010; Gorbunov 2011; Witt et al. 2011; Knyazev 2015; Knyazev & Zuban' 2016; Titov et al 2017). The Noctuoidea fauna of North East Kazakhstan is studied more or less satisfactorily, the summarizing paper has been published two years ago (Titov et al 2017). In this paper, the authors report 480 Noctuoidea species including 20 species of the tribe Arctiini.