Spontaneous Pneumothorax Can Be Associated with TGFBR2 Mutation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Challenges in Long-Term Control of Hypercalcaemia with Denosumab
Taylor-Miller, T., Sivaprakasam, P., Smithson, S. F., Steward, C. G., & Burren, C. P. (2021). Challenges in long-term control of hypercalcaemia with denosumab after haematopoietic stem cell transplantation for TNFRSF11A osteoclast-poor autosomal recessive osteopetrosis. Bone reports, 14, 100738. [100738]. https://doi.org/10.1016/j.bonr.2020.100738 Publisher's PDF, also known as Version of record License (if available): CC BY-NC-ND Link to published version (if available): 10.1016/j.bonr.2020.100738 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Elsevier at https://doi.org/10.1016/j.bonr.2020.100738. Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Bone Reports 14 (2021) 100738 Contents lists available at ScienceDirect Bone Reports journal homepage: www.elsevier.com/locate/bonr Case Report Challenges in long-term control of hypercalcaemia with denosumab after haematopoietic stem cell transplantation for TNFRSF11A osteoclast-poor autosomal recessive osteopetrosis Tashunka Taylor-Miller a, Ponni Sivaprakasam b, Sarah F. Smithson c,d, Colin G. Steward d,e, Christine P. Burren a,d,* a Department of Paediatric -
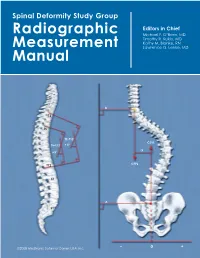
Spinal Deformity Study Group
Spinal Deformity Study Group Editors in Chief Radiographic Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Measurement Lawrence G. Lenke, MD Manual B T2 T5 T2–T12 CSVL T5–T12 +X° -X +X° C7PL T12 L2 A S1 ©2008 Medtronic Sofamor Danek USA, Inc. – 0 + Radiographic Measurement Manual Editors in Chief Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Lawrence G. Lenke, MD Section Editors Keith H. Bridwell, MD Kathy M. Blanke, RN Christopher L. Hamill, MD William C. Horton, MD Timothy R. Kuklo, MD Hubert B. Labelle, MD Lawrence G. Lenke, MD Michael F. O’Brien, MD David W. Polly Jr, MD B. Stephens Richards III, MD Pierre Roussouly, MD James O. Sanders, MD ©2008 Medtronic Sofamor Danek USA, Inc. Acknowledgements Radiographic Measurement Manual The radiographic measurement manual has been developed to present standardized techniques for radiographic measurement. In addition, this manual will serve as a complimentary guide for the Spinal Deformity Study Group’s radiographic measurement software. Special thanks to the following members of the Spinal Deformity Study Group in the development of this manual. Sigurd Berven, MD Hubert B. Labelle, MD Randal Betz, MD Lawrence G. Lenke, MD Fabien D. Bitan, MD Thomas G. Lowe, MD John T. Braun, MD John P. Lubicky, MD Keith H. Bridwell, MD Steven M. Mardjetko, MD Courtney W. Brown, MD Richard E. McCarthy, MD Daniel H. Chopin, MD Andrew A. Merola, MD Edgar G. Dawson, MD Michael Neuwirth, MD Christopher DeWald, MD Peter O. Newton, MD Mohammad Diab, MD Michael F. -

Lordosis, Kyphosis, and Scoliosis
SPINAL CURVATURES: LORDOSIS, KYPHOSIS, AND SCOLIOSIS The human spine normally curves to aid in stability or balance and to assist in absorbing shock during movement. These gentle curves can be seen from the side or lateral view of the spine. When viewed from the back, the spine should run straight down the middle of the back. When there are abnormalities or changes in the natural spinal curvature, these abnormalities are named with the following conditions and include the following symptoms. LORDOSIS Some lordosis is normal in the lower portion or, lumbar section, of the human spine. A decreased or exaggerated amount of lordosis that is causing spinal instability is a condition that may affect some patients. Symptoms of Lordosis include: ● Appearance of sway back where the lower back region has a pronounced curve and looks hollow with a pronounced buttock area ● Difficulty with movement in certain directions ● Low back pain KYPHOSIS This condition is diagnosed when the patient has a rounded upper back and the spine is bent over or curved more than 50 degrees. Symptoms of Kyphosis include: ● Curved or hunched upper back ● Patient’s head that leans forward ● May have upper back pain ● Experiences upper back discomfort after movement or exercise SCOLIOSIS The most common of the three curvatures. This condition is diagnosed when the spine looks like a “s” or “c” from the back. The spine is not straight up and down but has a curve or two running side-to-side. Sagittal Balance Definition • Sagittal= front-to-back direction (sagittal plane) • Imbalance= Lack of harmony or balance Etiology • Excessive lordosis (backwards lean) or kyphosis (forward lean) • Traumatic injury • Previous spinal fusion that disrupted sagittal balance Effects • Low back pain • Difficulty walking • Inability to look straight ahead when upright The most ergonomic and natural posture is to maintain neutral balance, with the head positioned over the shoulders and pelvis. -

[email protected] Patient Information Patient Name (Last, First, Middle) Birth Date (Mm-Dd-Yyyy) Sex Male Female
Marfan and Related Disorders Patient Information Instructions: The accurate interpretation and reporting of the genetic results is contingent upon the reason for referral, clinical information, ethnic background, and family history. To help provide the best possible service, supply the information requested below and send paperwork with the specimen, or return by fax to Mayo Clinic Laboratories, Attn: Personalized Genomics Laboratory Genetic Counselors at 507-284-1759. Phone: 507-266-5700 / International clients: +1-507-266-5700 or email [email protected] Patient Information Patient Name (Last, First, Middle) Birth Date (mm-dd-yyyy) Sex Male Female Referring Provider Name (Last, First) Phone Fax* Other Contact Name (Last, First) Phone Fax* *Fax number given must be from a fax machine that complies with applicable HIPAA regulations. Is this a postmortem specimen? Yes No If yes, attach autopsy report if available. Clinical History Reason for Testing (Check all that apply.) Diagnosis Carrier testing Presymptomatic diagnosis Family history Sudden death Note: Genetic testing should always be initiated on an affected family member first, if possible, in order to be most informative for at-risk relatives. See Ethnic Background and Family History section for more information. Diagnosis/Suspected Diagnosis Marfan Syndrome Ehlers-Danlos Syndrome Loeys-Dietz Syndrome Familial thoracic aortic aneurysm and dissection Other: _______________________________________________________________________________________________ Indicate whether the following -

Kyphectomy in Neonates with Meningomyelocele
Child's Nervous System (2019) 35:673–681 https://doi.org/10.1007/s00381-018-4006-4 ORIGINAL PAPER Kyphectomy in neonates with meningomyelocele Nail Özdemir1 & Senem Alkan Özdemir2 & Esra Arun Özer3 Received: 18 August 2018 /Accepted: 18 November 2018 /Published online: 11 December 2018 # Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Purpose Kyphosis is the most severe spinal deformity associated with meningomyelocele (MMC) and is seen in approximately 15% of neonates. Our purpose is to present our clinical experience, to discuss the technique and deformity correction in kyphectomy in neonates with MMC, and to assess its long-term outcomes. Method In this prospective study, the authors reviewed eight cases submitted to surgery between 2013 and 2015. We evaluated clinical characteristics that were analyzed, as were the operative technique employed, and angle range of the kyphosis deformity postcorrection follow-up. Results Neonatal kyphectomy was performed of six females and two males. The mean birth weight was 2780 g, and the mean age at the time of surgery was 5.6 days. There were S-shaped type deformity in lumbar region in all neonates. In the correction of the kyphotic deformity, a total vertebrae were removed from four patient, whereas a partial vertebrectomy was done in four. The mean operative time was 116 min. No patients did not require the blood transfusion. There were no serious complications, and wound closure was successful in all patients. The mean follow-up period was 4 years and 3 months (range 36–61 months), except one patient who died 1 week after discharge. -

Scoliosis in Paraplegia
Paraplegia (1974), II, 290-292 SCOLIOSIS IN PARAPLEGIA By JOHN A. ODOM, JR., M.D. and COURTN EY W. BROWN, M.D. Children's Hospital, Denver, Colorado in conjunction with ROBERT R. JACKSON, M.D., HARRY R. HA HN, M.D. and TERRY V. CARLE, M.D. Craig Rehabilitation Hospital, Englewood, Colorado WITH the increasing instance of excellent medical care, more children with traumatic paraplegia and myelomeningocele with paraplegia live to adulthood. In these two groups of patients there is a high instance of scoliosis, kyphosis and lordosis. Much attention in the past years has been placed on hips and feet but only in the last decade has there been much attention concentrated on the treatment of the spines of these patients. Most of this attention has been toward the patient with a traumatic paraplegia with development of scoliosis. Some attempts have been made at fusing the scoliotic spine of myelomeningo celes by Harrington Instrumentation but with a rather high instance of complica tions and failures. With the event of anterior instrumentation by Dr. Alan Dwyer of Sydney, Australia, the anterior approach to the spine is becoming widely accepted and very successfully used. This is especially valuable in the correction and fusion of the spine with no posterior elements from birth. MATERIAL FOR STUDY Between March of 1971 and June of 1973, there have been 26 paraplegics with scoliosis who have been cared for by the authors. All of these patients have either been myelomeningoceles with paraplegia or spinal cord injuries, all of whom have had scoliosis, kyphosis, or severe lordosis. -

Sotos Syndrome
European Journal of Human Genetics (2007) 15, 264–271 & 2007 Nature Publishing Group All rights reserved 1018-4813/07 $30.00 www.nature.com/ejhg PRACTICAL GENETICS In association with Sotos syndrome Sotos syndrome is an autosomal dominant condition characterised by a distinctive facial appearance, learning disability and overgrowth resulting in tall stature and macrocephaly. In 2002, Sotos syndrome was shown to be caused by mutations and deletions of NSD1, which encodes a histone methyltransferase implicated in chromatin regulation. More recently, the NSD1 mutational spectrum has been defined, the phenotype of Sotos syndrome clarified and diagnostic and management guidelines developed. Introduction In brief Sotos syndrome was first described in 1964 by Juan Sotos Sotos syndrome is characterised by a distinctive facial and the major diagnostic criteria of a distinctive facial appearance, learning disability and childhood over- appearance, childhood overgrowth and learning disability growth. were established in 1994 by Cole and Hughes.1,2 In 2002, Sotos syndrome is associated with cardiac anomalies, cloning of the breakpoints of a de novo t(5;8)(q35;q24.1) renal anomalies, seizures and/or scoliosis in B25% of translocation in a child with Sotos syndrome led to the cases and a broad variety of additional features occur discovery that Sotos syndrome is caused by haploinsuffi- less frequently. ciency of the Nuclear receptor Set Domain containing NSD1 abnormalities, such as truncating mutations, protein 1 gene, NSD1.3 Subsequently, extensive analyses of missense mutations in functional domains, partial overgrowth cases have shown that intragenic NSD1 muta- gene deletions and 5q35 microdeletions encompass- tions and 5q35 microdeletions encompassing NSD1 cause ing NSD1, are identifiable in the majority (490%) of 490% of Sotos syndrome cases.4–10 In addition, NSD1 Sotos syndrome cases. -

Differential Diagnosis Functional Vs Structural Scoliosis
Differential Diagnosis Functional vs Structural Scoliosis James J. Lehman, DC, MBA, FACO Associate Professor of Clinical Sciences University of Bridgeport College of Chiropractic Director Community Health Clinical Education University of Bridgeport Diagnosis is the key to successful treatment Scoliosis Classification Based upon the findings with this postural presentation, what physical examination procedures would you perform to determine your working diagnosis for the child with scoliosis? Classification of Scoliosis Structural or Nonstructural (functional) 1. Structural curves are fixed, nonflexible, and fail to correct with bending. 2. Nonstructural curves are not fixed but flexible and readily correct with bending. Postural Evaluation of Spine • Observation of standing posture • Right thoracic curve is most common with best prognosis Adam’s Position Differential Diagnosis Functional Scoliosis/Postural Imbalance Pelvic Obliquity and Postural Imbalance • You must determine whether the leg length discrepancy is anatomical or functional Actual Leg-Length Test • This is a tape measurement that tests for anatomical leg length discrepancy. • ASIS and medial malleolus are the landmarks identified Apparent Leg-Length Test • Reveals functional leg length discrepancy • Umbillicus and medial malleolus are landmarks • Evans Functional Leg-Length Measurement • Measure length of both lower extremities supine and seated • Inferior medial malloli are used as landmarks • Read the body language Functional Leg-Length Measurement • Usually the ipsilateral malleolus will measure short when supine if the superior iliac crest appears inferior when standing and long when seated Clinical Value of Long Sit Test • Pelvic Obliquity • Leg Length Discrepancy – Functional – Anatomical • SIJ Dysfunction • Spinal manipulation VIDEO: SUPINE TO LONG SIT TEST HTTP://WWW.THESTUDENTPHYSICALTHERAPIST.COM/SUPINE-TO-LONG- SIT-TEST.HTML LEVANGIE PK. -

Reliability of Measuring Thoracic Kyphosis Angle, Lumbar Lordosis Angle and Straight Leg Raise with an Inclinometer
10 The Open Spine Journal, 2012, 4, 10-15 Open Access Reliability of Measuring Thoracic Kyphosis Angle, Lumbar Lordosis Angle and Straight Leg Raise with an Inclinometer Andrew S. Van Blommestein1, Jeremy S. Lewis2,3,4, Matthew C. Morrissey5, and Sian MacRae1,2,* 1Division of Applied Biomedical Research, School of Biomedical and Health Sciences; 2Therapy Department, Chelsea and Westminster NHS Foundation Trust, London, UK; and 3Physiotherapy Department, St George's Healthcare NHS Trust, London, UK; 4Musculoskeletal Services, Central London Community Healthcare, London, UK; 5Faculty of Health Sciences, University of Ljubljana, Slovenia Abstract: Purpose: Several non-invasive measurement methods have been described in the literature for recording thoracic kyphosis, lumbar lordosis and straight leg raise (SLR). However, attempts to quantify the reliability of the inclinometer in these measurements are scarce. In addition, existing reliability studies within the literature were found to use small sample sizes. The aim of this investigation was to examine the intra-rater reliability of the chief investigator (SM), in order to provide clinicians with data that will allow them to better measure sagittal spinal posture and SLR. A blinded test-retest design was performed to determine the intra-rater reliability of thoracic kyphosis, lumbar lordosis and SLR when assessed using an Isomed inclinometer in normals. Methods. Thirty asymptomatic subjects were assessed on two occasions separated by a time interval of 1 hour to reduce investigator memory bias. Thoracic and lumbar measurements were recorded in a relaxed standing position using an inclinometer; SLR of the dominant leg was assessed with subjects in the supine position. Intraclass correlation coefficients (ICC), 95% confidence intervals (CI), and standard errors of measurement (SEM) were analysed to determine measurement reliability. -

Escobar Syndrome Associated with Spine and Orthopedic Pathologies
tics: Cu ne rr e en G t y R r e a Balioglu, Hereditary Genet 2015, 4:2 t s i e d a e r r c DOI: 10.4172/2161-1041.1000145 e h H Hereditary Genetics ISSN: 2161-1041 Case Report Open Access Escobar Syndrome Associated with Spine and Orthopedic Pathologies: Case Reports and Literature Review Balioglu MB* Metin Sabanci Baltalimani Bone Disease Education and Research Hospital, Istanbul, Turkey Abstract Escobar syndrome (ES) is associated with a web across every flexion crease in the extremities (most notably the popliteal space) and other structural anomalies such as a vertical talus, clubfoot, thoracic kyphoscoliosis and severe restrictive lung disease. In our study, we evaluated 3 patients diagnosed with multiple pterygium syndrome (MPS) type Escobar. The purpose of this study was to assess the abnormalities of the vertebrae and concomitant orthopedic pathologies. Two male patients (17 and 20-year-old siblings) and one female patient (9 year-old) were diagnosed with ES by genetic analysis. Patients had been diagnosed with kyphosis and progressive scoliosis (except one), high-set palate, ptosis, low-set ears, arachnodactyly, craniofacial dysmorphism, mild deafness, clubfoot, hip luxation, and joint contractures. Patients received operations for dislocation of the hip, clubfoot correction (except the female patient), and contractures of the knee and ankle. Furthermore, patients also underwent surgery for ptosis and inguinal hernias (except the female patient). One male patient received posterior vertebral instrumentation and fusion for a progressive spine deformity. Spinal and orthopedic pathologies commonly occur in patients with ES and scoliosis, and kyphosis may progress considerably over time. -

Klippel Feil Syndrome: a Case Report
Chattogram Maa-O-Shishu Hospital Medical College Journal Volume 19, Issue 1, January 2020 Case Report Klippel Feil Syndrome: A Case Report Dhananjoy Das1* Abstract M A Chowdhury (Arzu)1 Klippel-Feil Syndrome (KFS) is a complex syndrome comprises of classical clinical S M Zafar Hossain1 triad of short neck, limitation of head and neck movements and low posterior hairline. This syndrome is resulting from failure of the normal segmentation of cervical vertebra. 1Autism and Child Development Centre & In this present case in addition to classical clinical triad we have found short stature, Child Neurology Unit scoliosis at cervico- dorsal junction and sprengel deformity of the shoulder. We Chattogram Maa Shishu-O-Shishu Hospital Medical College didn’t find any association of hearing impairment, congenital heart disease and Chattogram, Bangladesh. renal abnormalities. There was no any neurological deficit and normal school performance. Patient with KFS usually have good prognosis if cardiopulmonary, genitourinary, auditory problems are identified and treated early. Key words: Congenital; Fusion; Klippel-Feil syndrome; Cervical vertebrae. INTRODUCTION Klippel-Feil Syndrome (KFS) was first discovered by Maurice Klippel and Andre Feil in 19121. KFS is a complex syndrome comprises of classical clinical triad of short neck, limitation of head and neck movements (Especially lateral bending) and low posterior hairline2. In 50% cases have all three component of this syndrome. It occurs in 1 of every 42,000 births and 60% cases are Female2. KF syndrome is group of deformities that result due to failure of the normal segmentation and fusion processes of mesodermal somites, which occurs between the third and seventh week of embryonic life3,4. -

Diastrophic Dysplasia
DIASTROPHIC DYSPLASIA Vernon Tolo, MD ICEOS 2018 DISCLOSURE • EDITOR EMERITUS, JBJS DIASTROPHIC DYSPLASIA – CHROMOSOMAL SITE 5q31-q34 – DEFECT IN DD SULFATE TRANSPORTASE – DIFFERENT GENOTYPES – RARE, EXCEPT IN FINLAND • CLINICAL FEATURES – SHORT, STIFF LIMBS – CLUBFEET – CAULIIFLOWER EAR – HITCHHIKER THUMB – VERY SHORT DIASTROPHIC DYSPLASIA – SPINAL ABNORMALITIES • CERVICAL KYPHOSIS • SEVERE KYPHOSCOLIOSIS • LUMBAR LORDOSIS AND STENOSIS DIASTROPHIC DYSPLASIA • CERVICAL SPINE KYPHOSIS – PRESENT AT BIRTH – AFFECTS C-3 TO C-5 – MOST RESOLVE WITHOUT TREATMENT • USUALLY BY AGE 6 YEARS – SMALL NUMBER WITH PROGRESSIVE KYPHOSIS • MAY DEVELOP MYELOPATHY DIASTROPHIC DYSPLASIA – CERVICAL KYPHOSIS UNTREATED – NEUROLOGIC NORMAL – DEVELOPMENTAL MILESTONES NORMAL FOR DD NEUTRAL FLEXION 2 YEARS 6 YEARS 6 DIASTROPHIC DYSPLASIA • NATURAL H/O CERVICAL KYPHOSIS (Remes, et al., 1999) – 120 PATIENTS IN FINLAND • NEWBORN TO 63 YEARS – 29 WITH KYPHOSIS OVERALL • 4/120 WITH SEVERE KYPHOSIS – 24/25 WITH XRAYS BY 18 MONTHS WITH KYPHOSIS • RESOLVED IN 24 BY MEAN 7.1 YEARS • KYPHOSIS < 60˚ SHOULD RESOLVE DIASTROPHIC DYSPLASIA • CERVICAL SPINE XRAY FINDINGS (Remes, et al. 2002) – 122 PATIENTS – AVERAGE LORDOSIS 17 DEGREES – FLAT VERTEBRAL BODIES – SAGITTAL CANAL NARROWED WITH AGE • DECLINE BEGINS AT AGE 8 YEARS – 79% WITH SPINA BIFIDA OCCULTA DIASTROPHIC DYSPLASIA • C-SPINE MRI FINDINGS (Remes, et al. 2000) – 90 PATIENTS AGED 3 MONTHS TO 50 YEARS • VERY WIDE FORAMEN MAGNUM • NARROWED SPINAL CANAL BELOW C-3 • ABNORMAL DISCS IN ALL, BEGINNING AT EARLY AGE – CERVICAL