Trichlorofluoromethane, Dichlorodifluoromethane, and Tetrachloromethane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Facile Procedure for the Generation of Dichlorocarbene from the Reaction of Carbon Tetrachloride and Magnesium Using Ultrasonic Irradiation
Molecules 2003, 8, 608-613 molecules ISSN 1420-3049 http://www.mdpi.org A Facile Procedure for the Generation of Dichlorocarbene from the Reaction of Carbon Tetrachloride and Magnesium using Ultrasonic Irradiation Haixia Lin *, Mingfa Yang, Peigang Huang and Weiguo Cao Department of Chemistry, Shanghai University, Shanghai, 200436, P.R. China *Author to whom correspondence should be addressed: e-mail [email protected] Received: 7 April 2003; in revised form: 7 July 2003 / Accepted: 20 July 2003 / Published: 31 July 2003 Abstract: An improved method for the generation of dichlorocarbene was developed that utilizes ultrasound in the reaction of carbon tetrachloride with magnesium. High yields of gem-dichlorocyclopropane derivatives can be obtained in the presence of olefins by this method. Keywords: Dichlorocarbene; gem-dichlorocyclopropanes; ultrasonic irradiation; olefin addition; magnesium Introduction Gem-dichlorocyclopropanes are valuable intermediates in organic synthesis [1,2]. They are typically prepared by the addition of dichlorocarbene to olefins under phase-transfer catalysis conditions [3-5]. Sonochemical generation of dichlorocarbene has also been reported [6-8]. The reactions of dichlorocarbene with olefins in solid-liquid two-phase systems using ultrasonication usually afford high yields of double-bond addition products. In addition, excellent yields of diadducts have been obtained from dienes and dichlorocarbene under ultrasonication and phase-transfer catalyst [9]. Molecules 2003, 8 609 Previously, we reported a novel route for the generation of dichlorocarbene by the reaction of carbon tetrachloride with magnesium in a neutral medium and hypothesized that the mechanism of these reactions might involve a single electron transfer [10]. However, these reactions suffered from several experimental drawbacks: some of the major ones being the sudden exotherm that occurs after an unpredictable induction period, foaming, and in some cases, the use of iodine as the activating agent. -
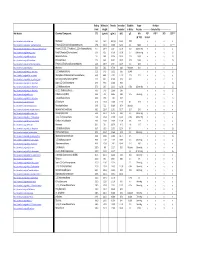
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

Dichlorodifluoromethane Dcf
DICHLORODIFLUOROMETHANE DCF CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99.5% (vol.) Common Synonyms Gas Colorless Faint odor Not flammable 7.2 Storage Temperature: Ambient Arcton 6 4.2 Flammable Limits in Air: Not flammable Eskimon 12 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not F-12 Visible vapor cloud is produced. 7.4 Venting: Safety relief flammable Freon 12 7.5 IMO Pollution Category: Currently not available Frigen 12 4.4 Fire Extinguishing Agents Not to Be Genetron 12 Used: Not flammable 7.6 Ship Type: Currently not available Halon 122 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: 3 Isotron 12 Products: Although nonflammable, Ucon 12 dissociation products generated in a fire may be irritating or toxic. 8. HAZARD CLASSIFICATIONS Notify local health and pollution control agencies. 4.6 Behavior in Fire: Helps extinguish fire. 8.1 49 CFR Category: Nonflammable gas Avoid inhalation. 4.7 Auto Ignition Temperature: Not 8.2 49 CFR Class: 2.2 flammable 8.3 49 CFR Package Group: Not pertinent. Not flammable. 4.8 Electrical Hazards: Not pertinent Fire Cool exposed containers with water. 8.4 Marine Pollutant: No 4.9 Burning Rate: Not flammable 8.5 NFPA Hazard Classification: Not listed 4.10 Adiabatic Flame Temperature: Currently 8.6 EPA Reportable Quantity: 5000 pounds CALL FOR MEDICAL AID. not available Exposure 8.7 EPA Pollution Category: D 4.11 Stoichometric Air to Fuel Ratio: Not VAPOR 8.8 RCRA Waste Number: U075 Not irritating to eyes, nose or throat. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

Chloroform 18.08.2020.Pdf
Chloroform Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested. Formula: CHCl₃ IUPAC ID: Trichloromethane Molar mass: 119.38 g/mol Boiling point: 61.2 °C Density: 1.49 g/cm³ Melting point: -63.5 °C The molecule adopts a tetrahedral molecular geometry with C3v symmetry. Chloroform volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl chloride, carbon monoxide, carbon dioxide, and hydrogen chloride. Its half-life in air ranges from 55 to 620 days. Biodegradation in water and soil is slow. Chloroform does not significantly bioaccumulate in aquatic organisms. Production:- In industry production, chloroform is produced by heating a mixture of chlorine and either chloromethane (CH3Cl) or methane (CH4). At 400–500 °C, a free radical halogenation occurs, converting these precursors to progressively more chlorinated compounds: CH4 + Cl2 → CH3Cl + HCl CH3Cl + Cl2 → CH2Cl2 + HCl CH2Cl2 + Cl2 → CHCl3 + HCl Chloroform undergoes further chlorination to yield carbon tetrachloride (CCl4): CHCl3 + Cl2 → CCl4 + HCl The output of this process is a mixture of the four chloromethanes (chloromethane, dichloromethane, chloroform, and carbon tetrachloride), which can then be separated by distillation. Chloroform may also be produced on a small scale via the haloform reaction between acetone and sodium hypochlorite: 3 NaClO + (CH3)2CO → CHCl3 + 2 NaOH + CH3COONa Deuterochloroform[ Deuterated chloroform is an isotopologue of chloroform with a single deuterium atom. -

Corynebacterium Sp.|NML98-0116
1 Limnochorda_pilosa~GCF_001544015.1@NZ_AP014924=Bacteria-Firmicutes-Limnochordia-Limnochordales-Limnochordaceae-Limnochorda-Limnochorda_pilosa 0,9635 Ammonifex_degensii|KC4~GCF_000024605.1@NC_013385=Bacteria-Firmicutes-Clostridia-Thermoanaerobacterales-Thermoanaerobacteraceae-Ammonifex-Ammonifex_degensii 0,985 Symbiobacterium_thermophilum|IAM14863~GCF_000009905.1@NC_006177=Bacteria-Firmicutes-Clostridia-Clostridiales-Symbiobacteriaceae-Symbiobacterium-Symbiobacterium_thermophilum Varibaculum_timonense~GCF_900169515.1@NZ_LT827020=Bacteria-Actinobacteria-Actinobacteria-Actinomycetales-Actinomycetaceae-Varibaculum-Varibaculum_timonense 1 Rubrobacter_aplysinae~GCF_001029505.1@NZ_LEKH01000003=Bacteria-Actinobacteria-Rubrobacteria-Rubrobacterales-Rubrobacteraceae-Rubrobacter-Rubrobacter_aplysinae 0,975 Rubrobacter_xylanophilus|DSM9941~GCF_000014185.1@NC_008148=Bacteria-Actinobacteria-Rubrobacteria-Rubrobacterales-Rubrobacteraceae-Rubrobacter-Rubrobacter_xylanophilus 1 Rubrobacter_radiotolerans~GCF_000661895.1@NZ_CP007514=Bacteria-Actinobacteria-Rubrobacteria-Rubrobacterales-Rubrobacteraceae-Rubrobacter-Rubrobacter_radiotolerans Actinobacteria_bacterium_rbg_16_64_13~GCA_001768675.1@MELN01000053=Bacteria-Actinobacteria-unknown_class-unknown_order-unknown_family-unknown_genus-Actinobacteria_bacterium_rbg_16_64_13 1 Actinobacteria_bacterium_13_2_20cm_68_14~GCA_001914705.1@MNDB01000040=Bacteria-Actinobacteria-unknown_class-unknown_order-unknown_family-unknown_genus-Actinobacteria_bacterium_13_2_20cm_68_14 1 0,9803 Thermoleophilum_album~GCF_900108055.1@NZ_FNWJ01000001=Bacteria-Actinobacteria-Thermoleophilia-Thermoleophilales-Thermoleophilaceae-Thermoleophilum-Thermoleophilum_album -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

Flammable Refrigerants Firefighter Training
Flammable refrigerants firefighter training: Hazard assessment and demonstrative testing FINAL REPORT BY: Noah L. Ryder, P. E. Stephen J. Jordan Fire & Risk Alliance, LLC. Rockville, Maryland, USA. Peter B. Sunderland, Ph.D. University of Maryland Department of Fire Protection Engineering College Park, Maryland, USA. May 2019 © 2019 Fire Protection Research Foundation 1 Batterymarch Park, Quincy, MA 02169-7417, USA Email: [email protected] | Web: nfpa.org/foundation ---page intentionally left blank--- —— Page ii —— FOREWORD The ongoing push toward sustainability of refrigeration systems will require the adoption of low global warming potential (GWP) refrigerants to meet the shift in environmental regulations. Fire safety is a lingering issue with the new age of flammable refrigerants being adopted and first responders may not be familiar with the change in material hazards or the appropriate response procedures required to safely handle these fire scenarios. This project is part of the overall two-year project with a goal to enhance firefighter safety and reduce potential injury by providing training on the hazards from appliances with flammable refrigerants. It will document the information about flammable refrigerants technologies and the hazards to emergency responders and develop interactive training modules to transfer the knowledge to the fire service. This report is focused to develop material documenting the hazards associated with flammable refrigerant technologies and the risks posed to the first responders. The material documentation included a literature review, Task 1, identifying baseline information on flammable refrigerants, their existing usage and implementation into products, potential integration into future technologies, and finally any existing guidance and best practices on response and tactics. -
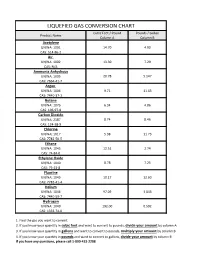
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Product Stewardship Summary Carbon Tetrachloride
Product Stewardship Summary Carbon Tetrachloride May 31, 2017 version Summary This Product Stewardship Summary is intended to give general information about Carbon Tetrachloride. It is not intended to provide an in-depth discussion of all health and safety information about the product or to replace any required regulatory communications. Carbon tetrachloride is a colorless liquid with a sweet smell that can be detected at low levels; however, odor is not a reliable indicator that occupational exposure levels have not been exceeded. Its chemical formula is CC14. Most emissive uses of carbon tetrachloride, a “Class I” ozone-depleting substance (ODS), have been phased out under the Montreal Protocol on Substances that Deplete the Ozone Layer (Montreal Protocol). Certain industrial uses (e.g. feedstock/intermediate, essential laboratory and analytical procedures, and process agent) are allowed under the Montreal Protocol. 1. Chemical Identity Name: carbon tetrachloride Synonyms: tetrachloromethane, methane tetrachloride, perchloromethane, benzinoform Chemical Abstracts Service (CAS) number: 56-23-5 Chemical Formula: CC14 Molecular Weight: 153.8 Carbon tetrachloride is a colorless liquid. It has a sweet odor, and is practically nonflammable at ambient temperatures. 2. Production Carbon tetrachloride can be manufactured by the chlorination of methyl chloride according to the following reaction: CH3C1+ 3C12 —> CC14 + 3HC1 Carbon tetrachloride can also be formed as a byproduct of the manufacture of chloromethanes or perchloroethylene. OxyChem manufactures carbon tetrachloride at facilities in Geismar, Louisiana and Wichita, Kansas. Page 1 of 7 3. Uses The use of carbon tetrachloride in consumer products is banned by the Consumer Product Safety Commission (CPSC), under the Federal Hazardous Substance Act (FHSA) 16 CFR 1500.17. -

Gas-Solid Alkali Destruction of Volatile Chlorocarbons
LA-13042-MS Gas-Solid Alkali Destruction of Volatile Chlorocarbons c£lVi •% to BO Los Alamos NATIONAL LABORATORY Los Alamos National Laboratory is operated by the University of California for the United States Department of Energy under contract W-7405-ENG-36- An Affirmative Action/Equal Opportunity Employer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither The Regents of the University of California, the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by The Regents of the University of California, the United States Government, or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of The Regents of the University of California, the United States Government, or any agency thereof. The Los Alamos National Laboratory strongly supports academic freedom and a researcher's right to publish; therefore, the Laboratory as an institution does not endorse the viewpoint of a publication or guarantee its technical correctness. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best aTaiiable original document. LA-13042-MS UC-901 Issued: December 1995 Gas-Solid Alkali Destruction of Volatile Chlorocarbons Jerry Foropoulos, Jr.