Umpolung: Carbonyl Synthons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sep. 5, Hydroacylation by Brandon Reinus
Hydroacylation and Related Topics Dong Group Seminar Brandon Reinus Wed, Sept. 5th 2012 Why? ¡ Looking at the reaction, it is a highly atom- economical approach to synthesizing ketones ¡ Umpolung (ex: deprotonating dithioacetals) ¡ Using acrylate derivatives generates a 1,4 diketone relationship, a hard relationship to establish using classical organic synthesis. Presentation Overview 1. Hydroformylation (extremely brief) 2. Rh-Catalyzed Hydroacylation ¡ Intramolecular ¡ Intermolecular ¡ Other 3. NHC Catalyzed Hydroacylation ¡ Benzoin reaction ¡ Stetter reaction ¡ other Part 1 : Background Reppe Roelen Science of Synthesis, Stereoselective Synthesis 1, 2011, pg.409 Hydroformylation Metal-Organic Cooperative Catalysis Park et al. Introduction SCHEME 2 Among the many elegant examples of transition metal cat- alyzed activation of C-HandC-Cbonds,1 chelation-as- sisted protocols have recently attracted increasing attention in organometallic chemistry. Directed metalation processes have been demonstrated by valuable applications in organic synthesis, showing remarkable efficiency and chemoselectivity.2 In general, a chelation-assisted proto- col facilitates the formation of either the kinetically or ther- modynamically favored five- or six-membered metallacycle; aprepositionedcoordinatinggroupinducesspatialproxim- ing decarbonylation, their structures are too specific to apply ity between the C-HorC-Cbondsandthetransitionmetal for common aldehydes. center.1,2 Despite the magnificent usefulness in activating otherwise stable C-HandC-Cbonds,amajordrawbackof -

(Nitroaldol) Reaction
MICROREVIEW DOI: 10.1002/ejoc.201101840 Biocatalytic Approaches to the Henry (Nitroaldol) Reaction Sinéad E. Milner,[a] Thomas S. Moody,[b] and Anita R. Maguire*[c] Keywords: Enzyme catalysis / Biocatalysis / C–C coupling / Nitroaldol reaction / Nitro alcohols Enantiopure β-nitro alcohols are key chiral building blocks approaches to the Henry (nitroaldol) reaction. The first for the synthesis of bioactive pharmaceutical ingredients. method is a direct enzyme-catalysed carbon–carbon bond The preparation of these target compounds in optically pure formation resulting in either an enantio-enriched or enantio- form has been the focus of much research and there has been pure β-nitro alcohol. The second approach describes the an emergence of biocatalytic protocols in the past decade. Henry reaction without stereocontrol followed by a biocata- For the first time, these biotransformations are the focus of lytic resolution to yield the enantiopure β-nitro alcohol. this review. Herein, we describe two principal biocatalytic Introduction The construction of carbon–carbon bonds is an essential element of synthetic organic chemistry. Among the various C–C bond forming reactions, the nitroaldol or Henry reac- tion[1] is one of the classical named reactions in organic synthesis. Essentially, this reaction describes the coupling of a nucleophilic nitro alkane with an electrophilic aldehyde or ketone to produce a synthetically useful β-nitro alcohol (Scheme 1).[2–5] Moreover, the Henry reaction facilitates the joining of two molecular fragments, under mild reaction conditions with the potential formation of two new ste- reogenic centres and a new C–C bond. The resulting β-nitro alcohols can undergo a variety of useful chemical transfor- mations which lead to synthetically useful structural motifs, e.g. -

UMPOLUNG in REACTIONS CATALYZED by THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung En Reacciones Catalizadas Por Enzimas Dependientes De Pirofosfato De Tiamina
Ciencia, Ambiente y Clima, Vol. 2, No. 2, julio-diciembre, 2019 • ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 DOI: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 UMPOLUNG IN REACTIONS CATALYZED BY THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung en reacciones catalizadas por enzimas dependientes de pirofosfato de tiamina Carlos José Boluda Emily Soto Instituto Tecnológico de Santo Domingo (INTEC), Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales, Av. de Los Área de Ciencias Básicas y Ambientales Próceres 49, Santo Domingo, República Dominicana Correo-e: [email protected] *Corresponding author: Carlos J. Boluda Darah de la Cruz Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Carolina Juncá Área de Ciencias Básicas y Ambientales Instituto Tecnológico de Santo Domingo (INTEC), Correo-e: [email protected] Área de Ciencias Básicas y Ambientales Anny Peña Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales Correo-e: [email protected] Recibido: 25/9/2019 • Aprobado: 19/10/2019 Cómo citar: Boluda, C. J., Juncá, C., Soto, E., de la Cruz, D., & Peña, A. (2019). Umpolung in reactions catalyzed by thiamine pyrophos- phate dependent enzymes. Ciencia, Ambiente Y Clima, 2(2), 27-42. Doi: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 Abstract Resumen The temporal exchange of the electrophilic/nucleophilic El intercambio temporal del carácter electrofílico/nucleofí- character of an atom by chemical manipulation is known lico de un átomo mediante manipulación química, es cono- in organic chemistry as umpolung. This inversion of polarity cido con el vocablo alemán de umpolung. -

Nitroso and Nitro Compounds 11/22/2014 Part 1
Hai Dao Baran Group Meeting Nitroso and Nitro Compounds 11/22/2014 Part 1. Introduction Nitro Compounds O D(Kcal/mol) d (Å) NO NO+ Ph NO Ph N cellular signaling 2 N O N O OH CH3−NO 40 1.48 molecule in mammals a nitro compound a nitronic acid nitric oxide b.p = 100 oC (8 mm) o CH3−NO2 57 1.47 nitrosonium m.p = 84 C ion (pKa = 2−6) CH3−NH2 79 1.47 IR: υ(N=O): 1621-1539 cm-1 CH3−I 56 Nitro group is an EWG (both −I and −M) Reaction Modes Nitro group is a "sink" of electron Nitroso vs. olefin: e Diels-Alder reaction: as dienophiles Nu O NO − NO Ene reaction 3 2 2 NO + N R h 2 O e Cope rearrangement υ O O Nu R2 N N N R1 N Nitroso vs. carbonyl R1 O O O O O N O O hυ Nucleophilic addition [O] N R2 R O O R3 Other reaction modes nitrite Radical addition high temp low temp nitrolium EWG [H] ion brown color less ion Redox reaction Photochemical reaction Nitroso Compounds (C-Nitroso Compounds) R2 R1 O R3 R1 Synthesis of C-Nitroso Compounds 2 O R1 R 2 N R3 3 R 3 N R N R N 3 + R2 2 R N O With NO sources: NaNO2/HCl, NOBF4, NOCl, NOSbF6, RONO... 1 R O R R1 O Substitution trans-dimer monomer: blue color cis-dimer colorless colorless R R NOBF OH 4 - R = OH, OMe, Me, NR2, NHR N R2 R3 = H or NaNO /HCl - para-selectivity ΔG = 10 Kcal mol-1 Me 2 Me R1 NO oxime R rate determining step Blue color: n π∗ absorption band 630-790 nm IR: υ(N=O): 1621-1539 cm-1, dimer υ(N−O): 1300 (cis), 1200 (trans) cm-1 + 1 Me H NMR (α-C-H) δ = 4 ppm: nitroso is an EWG ON H 3 Kochi et al. -
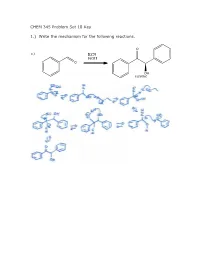
CHEM 345 Problem Set 18 Key 1.) Write the Mechanism for The
CHEM 345 Problem Set 18 Key 1.) Write the mechanism for the following reactions. O a.) KCN EtOH O OH racemic 1.) Write the mechanism for the following reactions. b.) KCN AcOH O NC OH racemic O c.) N S R O NEt3 OH racemic 2.) What is the structure of AcOH?Why does changing the solvent from EtOH to AcOH make such a big difference? O OH AcOH acetic acid The pKa of acetic acid is approximately 5. The pKa of ethanol is approximately 15. When you take a proton off of ethanol, you generate ethoxide which is about 1010 times stronger of a base than acetate. 3.) Give two instances when you need to use the thiazolium salt and triethylamine rather than KCN and EtOH. If the aldehydes contain an enolizable proton then you cannot use KCN/EtOH, instead you must use the thiazolium. Also, if the electrophile is a Michael acceptor to give a 1,4 dicarbonyl, then the thiazolium catalyst should be used. 4.) Break the following compound down as far as you can using Aldol, Michael, and Claisen reactions. Above each retrosynthetic arrow, write the name of the reaction. O HO O HO Aldol O Michael HO O O HO O Aldol O O Aldol HO O O HO Michael O O O Aldol There are other possibilities for order. O O HO Aldol O O 5.) Synthesize the following molecules. All carbons in the molecules must come from benzene or compounds with 5C’s or less. a.) O H2SO4 O HNO3 O2N AlCl3 O O SOCl2 HO Cl H2CrO4 1.) BuLi, Et2O + O 2.) H3O HO b.) O O O Cl + H3O NaOEt, EtOH O O O O Cl O AlCl3 Cl Cl AlCl3 Cl2 c.) O OMe NaOMe MeOH O O 1.) POCl3, DMF 2.) H2O OMe OMe O AlCl3 MeI Cl ONa HCl NaOH ZnHg 1.) NaOH + mcpba O 2.) H3O O OH O O AlCl3 Cl 6.) Write the mechanism for the following reactions. -
Title Several Reactions of Isocyanide and Related Compounds( Dissertation 全文 ) Author(S) Kobayashi, Shiro Citation
Several Reactions of Isocyanide and Related Compounds( Title Dissertation_全文 ) Author(s) Kobayashi, Shiro Citation 京都大学 Issue Date 1969-07-23 URL https://doi.org/10.14989/doctor.k916 Right Type Thesis or Dissertation Textversion author Kyoto University IN ISOCYANIDE AND COMPOUNDS 6 IR B s SEVERAL REACTIONS OF ISOCYANIDE AND RELATED COMPOUNDS 1 9 6 9 SHIRO KOBA Y ASHI PREFACE In the present thesis are collected the author's studies which have been carried out under the direction of Profossor Takeo Saegusa at Kyoto University during 1966 -- 1969. The studies include new organic reactions of isocyanide, carbon monoxide and carbene, which are characterized by a carbon atom carry ing lone-pair electrons. Reactions catalyzed by copper compounds as well as other Groups IB and IIB metal compounds constitute the central featl1re of tIl':' present studies. New organic reactions of isocyanidE' without catalysts are also presented. The author expresses his deep gratitude to Professor Takeo Saes'Usa for his constant guidance and encouragement throughout the work. Grateful acknowledgement is also made to Dr. Yoshihiko Ito for his valuable advice an? dis cussions during the course of studies. The author wishes to express his deep appreciation to Messrs. Kiwami Hirota, Nobuyuki Takeda, Toyoji Shimizu, Hiroshi Yoshioka, Yoshiharu Okumura. and Ikuo Morino for their active collaborations in carrying out the experiments. Shiro Kobayashi Department of Synthetic Chemistry Kyoto University March, 1969. (i) CON TEN TS Page INTRODUCTION ............................ .. 1 SYNOPSES .............................. .. 7 PART I. INSERTION REACTIONS OF ISOCYNJIDE CATALYZED BY COPPER COMPOUNDS 15 Chapter 1. Reaction of Isocyanide with Amine Catalyzed by Groups IB and IIB Metal Compounds, main ly by Copper Compounds. -

Benzoin and Stobbe Reactions
____________________________________________________________________________________________________ Subject Chemistry Paper No and Title 9; Organic Chemistry-III (Reaction Mechanism-2) Module No and Title 21; Named Reactions: Benzoin Condensation and Stobbe Condensation Module Tag CHE_P9_M21 CHEMISTRY Paper 9: Organic Chemistry-III (Reaction Mechanism-2) Module NO. 21: Benzoin Condensation and Stobbe Condensation ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Benzoin Condensation 3.1 Mechanism of Benzoin Condensation 3.2 Characteristics of Benzoin Condensation 3.3 Reactions of Benzoin 4. Stobbe Condensation 4.1 Mechanism of Stobbe Condensation 4.2 Characteristics of Stobbe Condensation 4.3 Few Examples of Stobbe Condensation 5. Summary CHEMISTRY Paper 9: Organic Chemistry-III (Reaction Mechanism-2) Module NO. 21: Benzoin Condensation and Stobbe Condensation ____________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to: Know what are Benzoin condensation and Stobbe condensation reactions Learn mechanism of Benzoin and Stobbe condensation reactions Know about the role of CN- ion in Benzoin condensation Identify the products of reduction and oxidation of Benzoin condensation Understand the product formation in Benzoin and Stobbe condensation. 2. Introduction A condensation reaction, also commonly referred to as dehydration -

UC San Diego UC San Diego Electronic Theses and Dissertations
UC San Diego UC San Diego Electronic Theses and Dissertations Title Isolation of Four-Coordinate Iridium(I) Monohydrides and the X-ray Crystal Structure of a Cobalt Tris-Isocyanide Alkane sigma-Complex / Permalink https://escholarship.org/uc/item/1b20p9sg Author Millard, Matthew David Publication Date 2013 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA, SAN DIEGO Isolation of Four-Coordinate Iridium(I) Monohydrides and the X-ray Crystal Structure of a Cobalt Tris-Isocyanide Alkane sigma-Complex A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry by Matthew David Millard Committee in charge: Professor Joshua S. Figueroa, Chair Professor Joseph M. O‘Connor Professor Nicholas C. Spitzer Professor F. Akif Tezcan Professor William C. Trogler 2013 Copyright Matthew David Millard, 2013 All rights reserved Ignature Page The dissertation of Matthew David Millard is approved, and it is acceptable in quality and form for publication on microfilm and electronically. Chair University of California, San Diego 2013 iii DEDICATION To my mother: for marrying the guy being chased by the cops while doing a wheelie on an orange motorbike up the street. iv EPIGRAPH Epig NEVER SAY NO TO A CHALLENGE Professor Karl O. Christe v TABLE OF CONTENTS Signature Page ......................................................................................................................... iii Dedication ............................................................................................................................... -

(Mcrs) of the Last Decade
Molecules 2012, 17, 1074-1102; doi:10.3390/molecules17011074 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Diversity Oriented Syntheses of Conventional Heterocycles by Smart Multi Component Reactions (MCRs) of the Last Decade Heiner Eckert Department Chemie, Technische Universität München, Lichtenbergstr. 4, Garching 85747, Germany; E-Mail: [email protected]; Tel.: +49-89-354-5532; Fax: +49-89-289-13329 Received: 9 December 2011; in revised form: 11 January 2012 / Accepted: 12 January 2012 / Published: 20 January 2012 Abstract: A collection of smart multicomponent reactions (MCRs) with continuative post condensation cyclizations (PCCs) is presented to construct conventional three- to seven- membered heterocyclic compounds in diversity oriented syntheses (DOS). These will provide a high degree of applying economical and ecological advantages as well as of practicability. Water, ionic liquids, and solvent-less syntheses as well as use of various forms of energy as microwave and ultrasonic irradiation are examined and discussed. Keywords: multicomponent reaction; MCR; MFCR; I-MCR; isocyanide; heterocycle; diversity oriented synthesis; solvent-less synthesis; alternative energy; microwave 1. Introduction Multi Component Reaction (MCR) chemistry [1,2] applied to the synthesis of heterocycles [3–5] with all its variations and extensions has undergone an enormous and meaningful upturn. Seeds sown in the last century, particularly appreciated by Ugi [6], have grown enormously and provided a plurality of novel reactions, new smart strategies as well as forward-looking methods, and high product diversity [7,8]. In this paper syntheses of conventional three to seven-membered heterocyclic structures (aziridine, azetidine, pyrrole, pyrrolidine, furan, indole, isoindoline, pyrazole, pyrazoline, imidazole, oxazolidine, thiazole, triazole, triazolidine, tetrazole, pyridine, pyrane, isoquinoline, pyrimidine, piperazine, oxazine, tetrazine, and oxadiazepine) are presented. -

Section I Haloakanes
SECTION I HALOAKANES Compounds derived from alkanes by the replacement of one or more Hydrogen atoms by corresponding number of halogen atoms ( fluorine, chlorine, bromine or iodine) are termed as haloalkanes. Alkyl halides are represented by general formula CnH2n+1X, here X is halogen atom ORBITAL STRUCTURE In alkyl halides, carbon-halogen σ bond is formed by overlap of sp3 hybrid orbital of carbon and half filled valence p-orbital of halogen atom: Haloalkanes may be classified on the basis of number of halogen atoms (1) Monohalogen derivatives One halogen atom is attached to carbon atom. Its general formula is CnH2n+1X Example CH3Cl ( methyl chloride). (2) Dihalogen derivatives These are derived by replacement of two hydrogen atoms by two halogen atoms Dihalides derivatives are of three types (a) Gem-dihalides Halogen atoms are attached to same carbon atom. These are called alkylidene halides. 1 of 23 pages www.spiroacademy.com HALOALKANES AND HALOARENES www.spiroacademy.com (b) Vic-dihalides Halogen atoms are attached to adjacent (vicinal) carbon atoms. These are termed as alkylene halides. (c) α – ω halides ( terminal halides) Halogen atoms are attached to terminal carbon atoms. These are also called polymethyl halides Br – CH2 – CH2 – CH2 - Br Trimethyl di bromide ( 1,3 – dibromopropane) (3) Trihalogen derivatives Trihalogen derivatives are derived by replacing three hydrogen atoms by three halogen atoms. General formula is CnH2n-1X 2 of 23 pages www.spiroacademy.com HALOALKANES AND HALOARENES www.spiroacademy.com CLASSIFICATION OF MONOHALOGEN COMPOUND (1) Alkyl halides are classified as primary 1O, secondary 2O, tertiary 3O depending upon nature of carbon to which halogen is attached (2) Compounds containing sp3 , C – X bond (a) Alkyl halides CH3 – CH2 – CH2 – Cl ( 1- chloropropane) (b) Allylic carbon Halogen atom attached to allylic carbon. -

Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— a Literature Review
Molecules 2013, 18, 11873-11903; doi:10.3390/molecules181011873 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— A Literature Review Sowmini Kumaran 1, Mulchand S. Patel 2 and Frank Jordan 1,* 1 Department of Chemistry, Rutgers University, Newark, NJ 07102, USA 2 Department of Biochemistry, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-973-353-5470; Fax: +1-973-353-1264. Received: 30 July 2013; in revised form: 14 September 2013 / Accepted: 16 September 2013 / Published: 26 September 2013 Abstract: The 2-oxoacid dehydrogenase complexes (ODHc) consist of multiple copies of three enzyme components: E1, a 2-oxoacid decarboxylase; E2, dihydrolipoyl acyl-transferase; and E3, dihydrolipoyl dehydrogenase, that together catalyze the oxidative decarboxylation of 2-oxoacids, in the presence of thiamin diphosphate (ThDP), coenzyme A 2+ + (CoA), Mg and NAD , to generate CO2, NADH and the corresponding acyl-CoA. The structural scaffold of the complex is provided by E2, with E1 and E3 bound around the periphery. The three principal members of the family are pyruvate dehydrogenase (PDHc), 2-oxoglutarate dehydrogenase (OGDHc) and branched-chain 2-oxo acid dehydrogenase (BCKDHc). In this review, we report application of NMR-based approaches to both mechanistic and structural issues concerning these complexes. These studies revealed the nature and reactivity of transient intermediates on the enzymatic pathway and provided site-specific information on the architecture and binding specificity of the domain interfaces using solubilized truncated domain constructs of the multi-domain E2 component in its interactions with the E1 and E3 components. -

John Ulric N E F
NATIONAL ACADEMY OF SCIENCES JOHN ULRIC N EF 1862—1915 A Biographical Memoir by M E L V I L L E L . W O L F R O M Any opinions expressed in this memoir are those of the author(s) and do not necessarily reflect the views of the National Academy of Sciences. Biographical Memoir COPYRIGHT 1960 NATIONAL ACADEMY OF SCIENCES WASHINGTON D.C. JOHN ULRIC NEF' June 14,1862-August 13,1915 BY MELVILLE L. WOLFROM OHN ULRIC NEF was a great pioneer in American chemistry. It was J he, along with Arthur Michael and Ira Remsen, who was mainly responsible for the transfer to the universities of the United States of the tenets of the actively growing science of organic chemistry from the laboratories of the great European universities of the time. Nef was a pioneer in theoretical organic chemistry, a great experimental- ist, and an inspiring trainer of men. His advanced students, the Ph.D. trainees, went into positions in the American universities, and espe- cially in the Middle West, determined to carry on the tradition of research. In the words of one: "We were determined to keep some research going if it were only to boil water." This establishment of chemical research in the American universities was carried out under the most difficult of conditions and with little support or understand- ing on the part of the administrators of these growing institutions, who mainly considered the science departments, in the liberal arts colleges, as units which cost a lot of money and produced results of doubtful cultural value.