Influenza Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A/58/307 General Assembly
United Nations A/58/307 General Assembly Distr.: General 22 August 2003 Original: English Fifty-eighth session Item 119 (a) of the provisional agenda* Human rights questions: implementation of human rights instruments Status of the International Covenant on Economic, Social and Cultural Rights, the International Covenant on Civil and Political Rights and the Optional Protocols to the International Covenant on Civil and Political Rights Report of the Secretary-General** Summary The General Assembly, by its resolution 2200 A (XXI) of 16 December 1966, adopted and opened for signature, ratification or accession the International Covenant on Economic, Social and Cultural Rights, the International Covenant on Civil and Political Rights and the First Optional Protocol to the International Covenant on Civil and Political Rights, and expressed the hope that the Covenants and the Optional Protocol would be signed, ratified or acceded to without delay. The Assembly also requested the Secretary-General to submit to it at its future sessions reports concerning the status of ratification of the Covenants and of the Optional Protocol. In response to that request, reports on the status of the International Covenants and the Optional Protocol have been submitted annually to the Assembly since its twenty-second session in 1967. Both Covenants and the Optional Protocol were opened for signature at New York on 19 December 1966. In accordance with their respective provisions,1 the International Covenant on Economic, Social and Cultural Rights entered into force on 3 January 1976, the International Covenant on Civil and Political Rights entered * A/58/150. ** The footnote requested by the General Assembly in resolution 58/248 was not included in the submission. -

The Labor Force Experience of Women from 'Generation X'
Women from ‘‘Generation X' The labor force experience of women from ‘Generation X’ Women aged 25 to 34 years in 2000 participated in the labor force in greater proportions, were more educated, earned more, and generally enjoyed more labor market benefits than their counterparts 25 years earlier; moreover, the “earnings gap” between young women and men narrowed substantially over the period Marisa DiNatale uring the 1960s and 1970s, legislation years had completed 4 or more years of col- and and changing social mores dramatically lege, compared with 18 percent 25 years Stephanie Boraas Daltered the choices young women had earlier. about their futures. Girls growing up during this • Young women have substantially closed period were influenced both by the conventions the “earnings gap” with their male coun- of their parents’ generation and by the new op- terparts since 1979 (the first year for which portunities that were becoming available to them. comparable earnings data are available In contrast, girls born in later years grew up in an from the CPS). They earned 82 percent as era in which women often were expected to com- much as young men in 2000 for full-time bine market work1 with family responsibilities. work, compared with 68 percent in 1979. Consequently, women who were aged 25 to 34 years in 2000 had a markedly different relation- • Married women aged 25 to 34 years—par- ship to the labor market than did their counter- ticularly those who had children—were far parts in 1975. more likely to be in the labor force in 2000 The first part of this article focuses on the than 25 years earlier. -

1. D) Amendments to Articles 34 and 55 of the Constitution of the World Health Organization Geneva, 22 May 1973 .ENTRY INTO
1. d) Amendments to articles 34 and 55 of the Constitution of the World Health Organization Geneva, 22 May 1973 ENTRY. INTO FORCE: 3 February 1977, in accordance with article 73 of the Constitution, for all members of the World Health Organization*. REGISTRATION: 3 February 1977, No. 221. STATUS: Parties* TEXT: United Nations, Treaty Series , vol. 1035, p. 315. Note: The amendments to articles 34 and 55 of the Constitution of the World Health Organization were adopted by the Twenty-sixth World Health Assembly by resolution WHA 26.37 of 22 May 1973. In accordance with article 73 of the Constitution, amendments come into force for all Members when adopted by a two- thirds vote of the Health Assembly and accepted by two-thirds of the Members in accordance with their respective constitutional processes. Following is the list of States which had accepted the amendments prior to the entry into force of the Amendments. *See chapter IX.1 for the complete list of Participants, Members of the World Health Organization, for which the above amendments are in force, pursuant to article 73 of the Constitution. Participant1 Acceptance(A) Participant1 Acceptance(A) Afghanistan..................................................28 Feb 1975 A El Salvador ..................................................17 Oct 1975 A Argentina ..................................................... 4 Oct 1976 A Eswatini .......................................................18 Nov 1975 A Australia.......................................................11 Mar 1975 A Ethiopia....................................................... -

Cy Martin Collection
University of Oklahoma Libraries Western History Collections Cy Martin Collection Martin, Cy (1919–1980). Papers, 1966–1975. 2.33 feet. Author. Manuscripts (1968) of “Your Horoscope,” children’s stories, and books (1973–1975), all written by Martin; magazines (1966–1975), some containing stories by Martin; and biographical information on Cy Martin, who wrote under the pen name of William Stillman Keezer. _________________ Box 1 Real West: May 1966, January 1967, January 1968, April 1968, May 1968, June 1968, May 1969, June 1969, November 1969, May 1972, September 1972, December 1972, February 1973, March 1973, April 1973, June 1973. Real West (annual): 1970, 1972. Frontier West: February 1970, April 1970, June1970. True Frontier: December 1971. Outlaws of the Old West: October 1972. Mental Health and Human Behavior (3rd ed.) by William S. Keezer. The History of Astrology by Zolar. Box 2 Folder: 1. Workbook and experiments in physiological psychology. 2. Workbook for physiological psychology. 3. Cagliostro history. 4. Biographical notes on W.S. Keezer (pen name Cy Martin). 5. Miscellaneous stories (one by Venerable Ancestor Zerkee, others by Grandpa Doc). Real West: December 1969, February 1970, March 1970, May 1970, September 1970, October 1970, November 1970, December 1970, January 1971, May 1971, August 1971, December 1971, January 1972, February 1972. True Frontier: May 1969, September 1970, July 1971. Frontier Times: January 1969. Great West: December 1972. Real Frontier: April 1971. Box 3 Ford Times: February 1968. Popular Medicine: February 1968, December 1968, January 1971. Western Digest: November 1969 (2 copies). Golden West: March 1965, January 1965, May 1965 July 1965, September 1965, January 1966, March 1966, May 1966, September 1970, September 1970 (partial), July 1972, August 1972, November 1972, December 1972, December 1973. -

Median and Average Sales Prices of New Homes Sold in United States
Median and Average Sales Prices of New Homes Sold in United States Period Median Average Jan 1963 $17,200 (NA) Feb 1963 $17,700 (NA) Mar 1963 $18,200 (NA) Apr 1963 $18,200 (NA) May 1963 $17,500 (NA) Jun 1963 $18,000 (NA) Jul 1963 $18,400 (NA) Aug 1963 $17,800 (NA) Sep 1963 $17,900 (NA) Oct 1963 $17,600 (NA) Nov 1963 $18,400 (NA) Dec 1963 $18,700 (NA) Jan 1964 $17,800 (NA) Feb 1964 $18,000 (NA) Mar 1964 $19,000 (NA) Apr 1964 $18,800 (NA) May 1964 $19,300 (NA) Jun 1964 $18,800 (NA) Jul 1964 $19,100 (NA) Aug 1964 $18,900 (NA) Sep 1964 $18,900 (NA) Oct 1964 $18,900 (NA) Nov 1964 $19,300 (NA) Dec 1964 $21,000 (NA) Jan 1965 $20,700 (NA) Feb 1965 $20,400 (NA) Mar 1965 $19,800 (NA) Apr 1965 $19,900 (NA) May 1965 $19,600 (NA) Jun 1965 $19,800 (NA) Jul 1965 $21,000 (NA) Aug 1965 $20,200 (NA) Sep 1965 $19,600 (NA) Oct 1965 $19,900 (NA) Nov 1965 $20,600 (NA) Dec 1965 $20,300 (NA) Jan 1966 $21,200 (NA) Feb 1966 $20,900 (NA) Mar 1966 $20,800 (NA) Apr 1966 $23,000 (NA) May 1966 $22,300 (NA) Jun 1966 $21,200 (NA) Jul 1966 $21,800 (NA) Aug 1966 $20,700 (NA) Sep 1966 $22,200 (NA) Oct 1966 $20,800 (NA) Nov 1966 $21,700 (NA) Dec 1966 $21,700 (NA) Jan 1967 $22,200 (NA) Page 1 of 13 Median and Average Sales Prices of New Homes Sold in United States Period Median Average Feb 1967 $22,400 (NA) Mar 1967 $22,400 (NA) Apr 1967 $22,300 (NA) May 1967 $23,700 (NA) Jun 1967 $23,900 (NA) Jul 1967 $23,300 (NA) Aug 1967 $21,700 (NA) Sep 1967 $22,800 (NA) Oct 1967 $22,300 (NA) Nov 1967 $23,100 (NA) Dec 1967 $22,200 (NA) Jan 1968 $23,400 (NA) Feb 1968 $23,500 (NA) Mar 1968 -

Bangkok, 27 March 1976 .ENTRY INTO FORCE: 25 February 1979, In
2. CONSTITUTION OF THE ASIA-PACIFIC TELECOMMUNITY Bangkok, 27 March 1976 ENTRY. INTO FORCE: 25 February 1979, in accordance with article 18. REGISTRATION: 25 February 1979, No. 17583. STATUS: Signatories: 18. Parties: 41.1 TEXT: United Nations, Treaty Series , vol. 1129, p. 3. Note: The Constitution of the Asia-Pacific Telecommunity was adopted on 27 March 1976 by resolution 163 (XXXII)2 of the Economic and Social Commission for Asia and the Pacific at its thirty-second session, which took place at Bangkok, Thailand, from 24 March 1976 to 2 April 1976. The Constitution was open for signature at Bangkok from 1 April 1976 to 31 October 1976 and at the Headquarters of the United Nations in New York from 1 November 1976 to 24 February 1979. Ratification, Ratification, Acceptance(A), Acceptance(A), Participant Signature Accession(a) Participant Signature Accession(a) Afghanistan..................................................12 Jan 1977 17 May 1977 Mongolia......................................................14 Aug 1991 a Australia.......................................................26 Jul 1977 26 Jul 1977 Myanmar......................................................20 Oct 1976 9 Dec 1976 Bangladesh................................................... 1 Apr 1976 22 Oct 1976 Nauru ........................................................... 1 Apr 1976 22 Nov 1976 Bhutan..........................................................23 Jun 1998 a Nepal............................................................15 Sep 1976 12 May 1977 Brunei Darussalam3 -

Sahara Occidental Western Sahara
COUR INTERNATIONALE DE JUSTICE RECUEIL DES ARRÊTS, AVIS CONSULTATIFS ET ORDONNANCES SAHARA OCCIDENTAL (REQUÊTE POUR AVIS CONSULTATIF) ORDONNANCE DU 22 MAI 1975 INTERNATIONAL COURT OF JUSTICE REPORTS OF JUDGMENTS, ADVISORY OPINIONS AND ORDERS WESTERN SAHARA (REQUEST FOR ADVISORY OPINION) ORDER OF 22 MAY 1975 Mode officiel de citation: Sahara occidental, ordonnance du 22 mai 1975, C.I.J. Reclieil 1975, p. 6. Official citation : Western Sahara, Order of 22 May 1975, I.C.J. Reports 1975, p. 6. sales numbe-41 1 No de vente: INTERNATIONAL COURT OF JUSTICE YEAR 1975 1975 22 May 22 May 1975 General List No. 61 WESTERN SAHARA (REQUEST FOR ADVISORY OPINION) ORDER Present: President LACHS; Vice-President AMMOUN;Judges FORSTER, GROS,BENGZON, PETRÉN, ONYEAMA, DILLARD, IGNACIO-PINTO, DE CASTRO,MOROZOV, JIMÉNEZ DE ARÉCHAGA,Sir Humphrey WALDOCK,NAGENDRA SINGH, RUDA; Registrar AQUARONE. Composed as above, After deliberation, Having regard to Articles 31 and 68 of the Statute of the Court, and to Articles 3, 87 and 89 of the Rules of Court, Having regard to resolution 3292 (XXIX) of the General Assenibly of the United Nations dated 13 December 1974 requesting the International Court of Justice to give an advisory opinion on the following questions: "1. Was Western Sahara (Rio de Oro and Sakiet El Hamra) at the time of colonization by Spain a territory belonging to no one (terra nullius)? If the answer to the first question is in the negative, II. What were the legal ties between this territory and the Kingdom of Morocco and the Mauritanian entity?" 4 Makes -
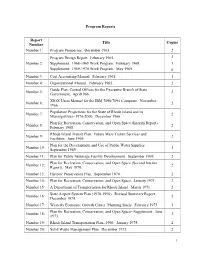
List of Technical Papers
Program Reports Report Title Copies Number Number 1: Program Prospectus. December 1963. 2 Program Design Report. February 1965. 2 Number 2: Supplement: 1968-1969 Work Program. February 1968. 1 Supplement: 1969-1970 Work Program. May 1969. 0 Number 3: Cost Accounting Manual. February 1965. 1 Number 4: Organizational Manual. February 1965. 2 Guide Plan: Central Offices for the Executive Branch of State Number 5: 2 Government. April1966. XIOX Users Manual for the IBM 7090/7094 Computer. November Number 6: 2 1966. Population Projections for the State of Rhode Island and its Number 7: 2 Municipalities--1970-2000. December 1966. Plan for Recreation, Conservation, and Open Space (Interim Report). Number 8: 2 February 1968. Rhode Island Transit Plan: Future Mass Transit Services and Number 9: 2 Facilities. June 1969. Plan for the Development and Use of Public Water Supplies. Number 10: 1 September 1969. Number 11: Plan for Public Sewerage Facility Development. September 1969. 2 Plan for Recreation, Conservation, and Open Space (Second Interim Number 12: 2 Report). May 1970. Number 13: Historic Preservation Plan. September 1970. 2 Number 14: Plan for Recreation, Conservation, and Open Space. January 1971. 2 Number 15: A Department of Transportation for Rhode Island. March 1971. 2 State Airport System Plan (1970-1990). Revised Summary Report. Number 16: 2 December 1974. Number 17: Westerly Economic Growth Center, Planning Study. February 1973. 1 Plan for Recreation, Conservation, and Open Space--Supplement. June Number 18: 2 1973. Number 19: Rhode Island Transportation Plan--1990. January 1975. 2 Number 20: Solid Waste Management Plan. December 1973. 2 1 Number 21: Report of the Trail Advisory Committee. -

POLLUTION CONTROL AGENCY: Solid and Hazardous Waste Division
MINNESOTA HISTORICAL SOCIETY Minnesota State Archives POLLUTION CONTROL AGENCY Solid and Hazardous Waste Division An Inventory of Its Project Grant Files OVERVIEW OF THE RECORDS Agency: Minnesota Pollution Control Agency. Division of Solid and Hazardous Waste. Series Title: Project grant files, Dates: 1973-1979. Abstract : Documentation of two grant-funded projects to develop procedures for the use and disposal of hazardous wastes. Quantity: 7.0 cu. ft. (7 boxes). Location: See Detailed Description section for box locations. SCOPE AND CONTENTS OF THE RECORDS Grant applications, work plans, progress reports, correspondence, and some background and publicity materials regarding two major projects carried out with grant funds from the U.S. Environmental Protection Agency. One was to conduct planning activities and develop rules for the use and disposal of hazardous wastes. The other was an attempt to construct and operate a demonstration chemical waste landfill facility, eventually terminated when no locally acceptable site could be found. findaids\state\pca013.inv POLLUTION CONTROL AGENCY. Solid and Hazardous Waste Division. Project Grant Files. p. 2 INDEX TERMS These records are indexed under the following headings in the catalog of the Minnesota Historical Society. Researchers desiring materials about related topics, persons or places should search the catalog using these headings. Topics: Hazardous waste disposal—Minnesota. Landfill siting—Minnesota. Waste disposal in the ground – Minnesota. Organizations: United States. Environmental Protection Agency. ADMINISTRATIVE INFORMATION Preferred Citation: [Indicate the cited volume and page or item and folder title here]. Minnesota Pollution Control Agency. Project Grant Files. Minnesota Historical Society. State Archives. See the Chicago Manual of Style for additional examples. -

No. 24384 MULTILATERAL Inter-American Convention on International Commercial Arbitration. Concluded at Panama City on 30 January
No. 24384 MULTILATERAL Inter-American Convention on international commercial arbitration. Concluded at Panama City on 30 January 1975 Authentic texts: Spanish, English, Portuguese and French. Registered by the Organization of American States on 23 October 1986. MULTILATERAL Convention interam ricaine sur l©arbitrage commercial international. Conclue Panama le 30 janvier 1975 Textes authentiques : espagnol, anglais, portugais et français. Enregistrée par l'Organisation des États américains le 23 octobre 1986. Vol. 1438,1-24384 1986 United Nations — Treaty Series • Nations Unies — Recueil des Traités_____249 INTER-AMERICAN CONVENTION1 ON INTERNATIONAL COM MERCIAL ARBITRATION The Governments of the Member States of the Organization of American States, desirous of concluding a convention on international commercial arbitration, have agreed as follows: Article 1. An agreement in which the parties undertake to submit to arbitral decision any differences that may arise or have arisen between them with respect to a commercial transaction is valid. The agreement shall be set forth in an instrument signed by the parties, or in the form of an exchange of letters, telegrams, or telex communications. Article 2. Arbitrators shall be appointed in the manner agreed upon by the parties. Their appointment may be delegated to a third party, whether a natural or juridical person. Arbitrators may be nationals or foreigners. Article 3. In the absence of an express agreement between the parties, the arbitration shall be conducted in accordance with the rules of procedure of the Inter-American Commercial Arbitration Commission. Article 4. An arbitral decision or award that is not appealable under the applicable law or procedural rules shall have the force of a final judicial judgment. -

General Agreement on Tariffs and Trade
CONFIDENTIAL GENERAL AGREEMENT ON MCDP/W/58/Rev.22 TARIFFS AND TRADE lU July 1978 Arrangement Concerning Certain Dairy Products MANAGEMENT COMMITTEE Information Required by the Committee under Article IV of the Arrangement Information under the Decision of 10 May 1976 Revision For the convenience of delegations . the secretariat has prepared and updated the following summary tables3 based on communications received to date in pursuance of the Decision of 10 May 1976. 4 5 INFORMATION RECEIVED IN PURSUANCE OF THE DECISION OF 10 MAY 1976 ~ Exporter Volume Destination Date of Pries Importer participants (n. tons) contract Delivery schedule Control measure conditions of sales Port of export Port of import Age of the powder attestation Australia 10,000 Romania October, November 1976 Melbourne/Portland Constantsa 1975 Filed Canada 1,200 Yugoslavia It. 5.1976 June 1976 l/3552/Add.6 Para.10 US$245 per ton c.l.f. Montreal Rijeka September/October/November 1974 Filed 175 Taiwan 18. 6.1976 July 1976 1/3552/Add.6 Para. 6 US$270 per 0.4 f. Montreal Keelung/Kaohslung July 1976 Filed (110 m.t. 1,250 Taiwan 18. 6.1976 July to October 1976 l/3552/*dd.6 Para. 6 US$270 per c.4 f. Montreal/St. John Kaohslung June 1976 Filed 100 Taiwan 23 6.1976 July, August 1976 l/3552/Add.6 Para. 6 US$270 per c.& f. St. John Kaohslung July 1976 Filed 5,000 Bulgaria 18. 6.1976 July, August 1976 l/3552/Add.6 Para.10 US$245 per c.4 f. Montreal Varna/Bougas April/July 1975 Filed 2,000 Bulgaria 11. -

D Thedi,. Cti,,Gco ,.Cll HEALTH
executive committee of u'orking party of _'?_/'-_'__dt_hedi,._cti,,gco_,.cll the reglonal comm,_te f_._ _ HEALTH _,¢_' t_ ORGANIZATION ORGANIZATION _-_ 76th Meeting Washington, D.C. June-July 1976 Provisional Agenda Item 5 CE76/5 (Eng.) 15 April 1976 ORIGINAL: ENGLISH-SPANISH REPORT ON THE COLLECTION OF QUOTA CONTRIBUTIONS The Director has the honor to present a report on the status of quota contributions as of 15 April 1976. In the Financial Report of the Director (Official Document 140) there is presented a table showing the status of quota comtributions as of 31 Decem- ber 1975. To provide the Executive Committee with the latest information, the corresponding updated table is attached. An examination of the table discloses that $290,460.90 in quota contri- butions has been received up to 15 April 1976. This compares with $606,002.72 received during this period in 1975 and $1,254,313.56 in 1974. Every effort is being made to urge Member Governments to make payment of quota arrears to avoid exhaustion of the Workimg Capital Fund. Annex CE76/5 (Eng.) ANNEX PAN AMERICAN HEALTH ORGANIZATION STATEMENT OF QUOTA CONTRIBUTIONS DUE FROM MEMBER GOVERNMENTS AND PARTICIPATING GOVERNMENTS 1976 BUDGET AND PRIOR YEARS (Expressed in U.S. dollars) Member Governments and Participating Amount Due Collected Balance Due Governments Year 1 January 1976 During 1976 15 April 1976 Argentina 1976 1,806,897.00 - 1,806,897.00 Bahamas 1976 14,650.00 6,030.00 8,620.00a Barbados 1976 19,534.00 1,969.00 17,565.00a Bolivia 1976 46,394.00 - 46,394.00 1975 41,049.00