1975 Air Quality Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cy Martin Collection
University of Oklahoma Libraries Western History Collections Cy Martin Collection Martin, Cy (1919–1980). Papers, 1966–1975. 2.33 feet. Author. Manuscripts (1968) of “Your Horoscope,” children’s stories, and books (1973–1975), all written by Martin; magazines (1966–1975), some containing stories by Martin; and biographical information on Cy Martin, who wrote under the pen name of William Stillman Keezer. _________________ Box 1 Real West: May 1966, January 1967, January 1968, April 1968, May 1968, June 1968, May 1969, June 1969, November 1969, May 1972, September 1972, December 1972, February 1973, March 1973, April 1973, June 1973. Real West (annual): 1970, 1972. Frontier West: February 1970, April 1970, June1970. True Frontier: December 1971. Outlaws of the Old West: October 1972. Mental Health and Human Behavior (3rd ed.) by William S. Keezer. The History of Astrology by Zolar. Box 2 Folder: 1. Workbook and experiments in physiological psychology. 2. Workbook for physiological psychology. 3. Cagliostro history. 4. Biographical notes on W.S. Keezer (pen name Cy Martin). 5. Miscellaneous stories (one by Venerable Ancestor Zerkee, others by Grandpa Doc). Real West: December 1969, February 1970, March 1970, May 1970, September 1970, October 1970, November 1970, December 1970, January 1971, May 1971, August 1971, December 1971, January 1972, February 1972. True Frontier: May 1969, September 1970, July 1971. Frontier Times: January 1969. Great West: December 1972. Real Frontier: April 1971. Box 3 Ford Times: February 1968. Popular Medicine: February 1968, December 1968, January 1971. Western Digest: November 1969 (2 copies). Golden West: March 1965, January 1965, May 1965 July 1965, September 1965, January 1966, March 1966, May 1966, September 1970, September 1970 (partial), July 1972, August 1972, November 1972, December 1972, December 1973. -
List of Technical Papers
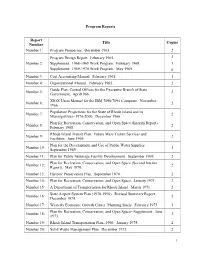
Program Reports Report Title Copies Number Number 1: Program Prospectus. December 1963. 2 Program Design Report. February 1965. 2 Number 2: Supplement: 1968-1969 Work Program. February 1968. 1 Supplement: 1969-1970 Work Program. May 1969. 0 Number 3: Cost Accounting Manual. February 1965. 1 Number 4: Organizational Manual. February 1965. 2 Guide Plan: Central Offices for the Executive Branch of State Number 5: 2 Government. April1966. XIOX Users Manual for the IBM 7090/7094 Computer. November Number 6: 2 1966. Population Projections for the State of Rhode Island and its Number 7: 2 Municipalities--1970-2000. December 1966. Plan for Recreation, Conservation, and Open Space (Interim Report). Number 8: 2 February 1968. Rhode Island Transit Plan: Future Mass Transit Services and Number 9: 2 Facilities. June 1969. Plan for the Development and Use of Public Water Supplies. Number 10: 1 September 1969. Number 11: Plan for Public Sewerage Facility Development. September 1969. 2 Plan for Recreation, Conservation, and Open Space (Second Interim Number 12: 2 Report). May 1970. Number 13: Historic Preservation Plan. September 1970. 2 Number 14: Plan for Recreation, Conservation, and Open Space. January 1971. 2 Number 15: A Department of Transportation for Rhode Island. March 1971. 2 State Airport System Plan (1970-1990). Revised Summary Report. Number 16: 2 December 1974. Number 17: Westerly Economic Growth Center, Planning Study. February 1973. 1 Plan for Recreation, Conservation, and Open Space--Supplement. June Number 18: 2 1973. Number 19: Rhode Island Transportation Plan--1990. January 1975. 2 Number 20: Solid Waste Management Plan. December 1973. 2 1 Number 21: Report of the Trail Advisory Committee. -

POLLUTION CONTROL AGENCY: Solid and Hazardous Waste Division
MINNESOTA HISTORICAL SOCIETY Minnesota State Archives POLLUTION CONTROL AGENCY Solid and Hazardous Waste Division An Inventory of Its Project Grant Files OVERVIEW OF THE RECORDS Agency: Minnesota Pollution Control Agency. Division of Solid and Hazardous Waste. Series Title: Project grant files, Dates: 1973-1979. Abstract : Documentation of two grant-funded projects to develop procedures for the use and disposal of hazardous wastes. Quantity: 7.0 cu. ft. (7 boxes). Location: See Detailed Description section for box locations. SCOPE AND CONTENTS OF THE RECORDS Grant applications, work plans, progress reports, correspondence, and some background and publicity materials regarding two major projects carried out with grant funds from the U.S. Environmental Protection Agency. One was to conduct planning activities and develop rules for the use and disposal of hazardous wastes. The other was an attempt to construct and operate a demonstration chemical waste landfill facility, eventually terminated when no locally acceptable site could be found. findaids\state\pca013.inv POLLUTION CONTROL AGENCY. Solid and Hazardous Waste Division. Project Grant Files. p. 2 INDEX TERMS These records are indexed under the following headings in the catalog of the Minnesota Historical Society. Researchers desiring materials about related topics, persons or places should search the catalog using these headings. Topics: Hazardous waste disposal—Minnesota. Landfill siting—Minnesota. Waste disposal in the ground – Minnesota. Organizations: United States. Environmental Protection Agency. ADMINISTRATIVE INFORMATION Preferred Citation: [Indicate the cited volume and page or item and folder title here]. Minnesota Pollution Control Agency. Project Grant Files. Minnesota Historical Society. State Archives. See the Chicago Manual of Style for additional examples. -

No. 24384 MULTILATERAL Inter-American Convention on International Commercial Arbitration. Concluded at Panama City on 30 January
No. 24384 MULTILATERAL Inter-American Convention on international commercial arbitration. Concluded at Panama City on 30 January 1975 Authentic texts: Spanish, English, Portuguese and French. Registered by the Organization of American States on 23 October 1986. MULTILATERAL Convention interam ricaine sur l©arbitrage commercial international. Conclue Panama le 30 janvier 1975 Textes authentiques : espagnol, anglais, portugais et français. Enregistrée par l'Organisation des États américains le 23 octobre 1986. Vol. 1438,1-24384 1986 United Nations — Treaty Series • Nations Unies — Recueil des Traités_____249 INTER-AMERICAN CONVENTION1 ON INTERNATIONAL COM MERCIAL ARBITRATION The Governments of the Member States of the Organization of American States, desirous of concluding a convention on international commercial arbitration, have agreed as follows: Article 1. An agreement in which the parties undertake to submit to arbitral decision any differences that may arise or have arisen between them with respect to a commercial transaction is valid. The agreement shall be set forth in an instrument signed by the parties, or in the form of an exchange of letters, telegrams, or telex communications. Article 2. Arbitrators shall be appointed in the manner agreed upon by the parties. Their appointment may be delegated to a third party, whether a natural or juridical person. Arbitrators may be nationals or foreigners. Article 3. In the absence of an express agreement between the parties, the arbitration shall be conducted in accordance with the rules of procedure of the Inter-American Commercial Arbitration Commission. Article 4. An arbitral decision or award that is not appealable under the applicable law or procedural rules shall have the force of a final judicial judgment. -

Band Returns in El Salvador, 1973-74 and 1974-75 Seasons
Vol.47, No. 3 Ge•eralNotes [277 begins in late July. By early to mid-September it has progressedto replacement of the fifth secondary. At that time all of the wing coverts and tertials will have been replaced, but not the ahfiae. Primaries eight and nine will have par- tially regrown at that time and much but not all of the body molt will be either complete or well under way. The geographical origin of the bird in question is not known, nor is the normal timing of its molt schedule. Therefore, its schedule may or may not coincide with that observed in Adirondack juncos. However, since this bird had none of the usual molt characteristicsthat accompany replacement of the last two secondariesand the alulae, apparently its wing molt had been stopped or suspendedshort of completion. This occurrenceis the first of its kind that I have observedin the handling of 180 Dark-eyed Juncosknown to be adult during the period of October through December in the past 12 years.--Ilom:•T P. Yu•cK, 1527 Myror• Street,Sche'•ectady, New York 12309. Received 25 January 1976, accepted 5 April 1976. An A•e Record for Swainson's Warbler.--l(ennard (Bird-Banding, 46: 55-73, 1975) published a summary of longevity records for North American birds but did not include one for Swainsoh'sWarbler (Lymnothlypisswainsonii). On 14 May 1960, Weske banded with •P31-27954 an AHY-U individual of this speciesin swampy woodland 7 km NNW of Whaleysville, Worcester Count.y, Maryland. The locality is at the extreme northern limit of the species' known breedingrange ou the Atlantic CoastalPlain (Meanley, N. -

Country Term # of Terms Total Years on the Council Presidencies # Of
Country Term # of Total Presidencies # of terms years on Presidencies the Council Elected Members Algeria 3 6 4 2004 - 2005 December 2004 1 1988 - 1989 May 1988, August 1989 2 1968 - 1969 July 1968 1 Angola 2 4 2 2015 – 2016 March 2016 1 2003 - 2004 November 2003 1 Argentina 9 18 15 2013 - 2014 August 2013, October 2014 2 2005 - 2006 January 2005, March 2006 2 1999 - 2000 February 2000 1 1994 - 1995 January 1995 1 1987 - 1988 March 1987, June 1988 2 1971 - 1972 March 1971, July 1972 2 1966 - 1967 January 1967 1 1959 - 1960 May 1959, April 1960 2 1948 - 1949 November 1948, November 1949 2 Australia 5 10 10 2013 - 2014 September 2013, November 2014 2 1985 - 1986 November 1985 1 1973 - 1974 October 1973, December 1974 2 1956 - 1957 June 1956, June 1957 2 1946 - 1947 February 1946, January 1947, December 1947 3 Austria 3 6 4 2009 - 2010 November 2009 1 1991 - 1992 March 1991, May 1992 2 1973 - 1974 November 1973 1 Azerbaijan 1 2 2 2012 - 2013 May 2012, October 2013 2 Bahrain 1 2 1 1998 - 1999 December 1998 1 Bangladesh 2 4 3 2000 - 2001 March 2000, June 2001 2 Country Term # of Total Presidencies # of terms years on Presidencies the Council 1979 - 1980 October 1979 1 Belarus1 1 2 1 1974 - 1975 January 1975 1 Belgium 5 10 11 2007 - 2008 June 2007, August 2008 2 1991 - 1992 April 1991, June 1992 2 1971 - 1972 April 1971, August 1972 2 1955 - 1956 July 1955, July 1956 2 1947 - 1948 February 1947, January 1948, December 1948 3 Benin 2 4 3 2004 - 2005 February 2005 1 1976 - 1977 March 1976, May 1977 2 Bolivia 3 6 7 2017 - 2018 June 2017, October -

Treaty Amending Certain Financial Provisions (22 July 1975)
Treaty amending Certain Financial Provisions (22 July 1975) Caption: Treaty amending certain financial provisions of the Treaties establishing the European Communities and of the Treaty establishing a Single Council and a Single Commission of the European Communities. On 22 July 1975, in Brussels, the Member States of the Europe of the Nine sign this Treaty which provides for the establishment of a Court of Auditors and strengthens the European Parliament's budgetary powers. These reforms proved necessary following the implementation of the financial system of own resources, which established the financial autonomy of the European Communities. Source: Official Journal of the European Communities (OJEC). 31.12.1977, n° L 359. [s.l.]. ISSN 0378-6978. "Treaty amending certain financial provisions of the Treaties establishing the European Economic Communities and of the Treaty establishing a single Council and a single Commission of the European Communities", p. 1. Copyright: All rights of reproduction, public communication, adaptation, distribution or dissemination via Internet, internal network or any other means are strictly reserved in all countries. The documents available on this Web site are the exclusive property of their authors or right holders. Requests for authorisation are to be addressed to the authors or right holders concerned. Further information may be obtained by referring to the legal notice and the terms and conditions of use regarding this site. URL: http://www.cvce.eu/obj/treaty_amending_certain_financial_provisions_22_july_1975-en-bd96621d-f5e8-4b9f- 9785-c5bd0f0cfed4.html Last updated: 02/04/2014 1 / 27 02/04/2014 Treaty amending certain financial provisions of the Treaties establishing the European Communities and of the Treaty establishing a single Council and a single Commission of the European Communities Chapter I - Provisions amending the Treaty establishing the European Coal and Steel Community ............. -

Flju~E0 __ \:...· ___---If
If you have issues viewing or accessing~- ~~ .... this"... ,.,-=-" file=",--~- contact us-------.--.,....,...,.-------- at NCJRS.gov. £1 VIOLENT CRIME National Criminal Justice Reference Service II IN ALBERTA~. ... i~1 PAPER' #3 I, " Parole and the Violent Offender tJ This microfiche was produced from documents received for inclusion in the NCJRS data base. Since NCJRS cannot exercise "'1,1 control over the physical condition of the documents submitted, , 1-1 the individual frame quality will vary. The resolution chart on this frame may be used to evaluate the document quality. I, I·IJ \~, (" 1 " '-t.-""_" I~ '1 : 111112,8 111\12.5 1 1 1 Ii& ~3,2 22 \\\\\1.0 ~ . f,f' L:: I~ ::tJ:.:. W .. ~ ,. Ill"" I \\\\\1.1 \ , nj IIIII~ IIIII~ 111111.6 IJ MICROCOPY RESOLUTION TEST CHART 11 1 J, NATIONAL BUREAU Of STANDARDS-1963-A I' iI i \ (' '/ Microfilming procedures used to create this fiche comply with the standards set forth in 41CFR 101-11.504. , l • 1 Points of view or opinions stated in this document are those of the author(s) and do notrepresent the official : ' ) ", ',' ," position or policies of the U. S. Department of Justice. ,? DATE FtlMtO ' ." J ~ : .. .i1:l'!~ti~iian~t~!'~flJU~e0 __\:.....· ____------If (} l United States Department of Justice 1 Washington, D. C. 20531 if ( I t.,. 1-4 ~•••••••••• _ 205 LEGISLATURE 1 I r the Official Opposttion' " .' 1 Ii: () I I' TABLE OF CONTENTS Page About the Authors ~ . i PAROLE A~D THE VIOLENT 'OFFENDER Acknowledgements ii Abstract iii c' By Public Perceptions of the Parole Board 1 The Implementation of Sentencing and Release Programs 1 Jim Hackler Remission and mandatory supervision 1 Q and Regular parole 2 i Laurel Gauld Day parole and.temporary absence m 3 .-"0'-'m .!!lc: 00"';: The Prediction of Violence and the Parole Board c:_ 3 O.c: .'" 0) (J'e "'> "00. -

GENERAL AGREEMENT on Niztjm? TARIFFS and TRADE Special Distribution
RESTRICTED GENERAL AGREEMENT ON niZTJm? TARIFFS AND TRADE Special Distribution Multilateral Trade Negotiations Group "Tariffs: TARIFF INFORMATION AVAILABLE IN THE SECRETARIAT Revision This document contains a revised and up-dated list of tariffs and their latest modifications available in the secretariat. The information, as noted here, is dravn from three sources: national editions of the customs tariffs, the publications of the International Customs Bureau in Brussels and the Handels-Archiv (published in Germany). MHT/TAR/W/5/Bev.2 Page 2 ANNEX Tariff Information Available in the Secretariat SOURCE Country Handels-Archiv Brussels Bureau National Tariff .AFGHANISTAN 1976 1955 modif. up to 1977 ALGERIA 1975 1973 1969 ANGOLA 1965 1967 modif. up to 1973 ARGENTINA 1977 1971 1968 AUSTRALIA 1972 1976 1976 (up to 1977) AUSTRIA 197U 1976 1972 (up to 1973) BAHAMAS 1977 1966 BAHREIN 1951+ BANGLADESH 1977 1973 BARBADOS 1976 1955 1966 MTN/TAR/W/5/Rev.2 Page 3 Country Handels-Archiv Brussels Bureau National Tariff BELGIUM 1969 1969 197T (Benelux) (Benelux) (Benelux) BENIN 1977 (WEST AFRICA) BOLIVIA 1976 197^ modif. up to 1977 BRAZIL 1973 1973 1977 BULGARIA 1968 1972 modif. up to 191k BURMA 1962 19^8 1967 modif. up to 1963 BURUNDI 1970 1975 modif. up to 197H CAMEROON 1977 1971 1966 (UDEAC) CANADA 1977 19lh 191h (up to 1977) OAPE VERDE 1966 CARIBBEAN 1976 COMMON MARKET MTN/TAR/W/5/Rev.2 Page k Country Handels-Archiv Brussels Bureau National Tariff CENTRAL AFRICAN 1977 1971 1969 EMPIRE (UDEAC) CENTRAL AMERICAN 1967 1969 COMMON MARKET modif. up to 1970 C:iÀD 1977 1971 1966 (UDEAC) CHILE 1976 197** 1967 nodif. -

GENERAL AGREEMENT on 28 September 1976 TARIFFS and TRADE Special Distribution
RESTRICTED MTN/TAR/W/5/Rev. 1 GENERAL AGREEMENT ON 28 September 1976 TARIFFS AND TRADE Special Distribution. Multilateral Trade Negotiations Group "Tariffs" TARIFF INFORMATION AVAILABLE IN THE SECRETARIAT Revision This document contains a revised and up-dated list of tariffs and their latest modifications available in the secretariat. The information, as noted here,is drawn mainly from three sources: national editions of the customs tariffs, the publications of the International Customs Bureau in Brussels and the Handels-Archiv (published in Germany). MTN/TAR/W/5/Rev.1 Page 2 ANNEX Tariff Information Available in the Secretariat SOURCE Country Handels-Archiv Brussels Bureau National Tariff AFGHANISTAN 1976 1955 ALGMRIA 1975 1973 1969 ARGENTINA 1966 1971 1968 modif. up to 1974 AUSTRALIA 1972 1976 1976 - Papua ) 1972 1957 (1969 - New Guinea) 1957 AUSTRIA 1974 1976 1972 (up to 1973) BAHAMAS 1961 1966 BAHRAIN 1954 BANGLADESH 1973 BARBADOS 1958 1955 1966 MTN/TAR/W/5/Rev.1 Page 3 Country Handels-Archiv Brussels Bureau National Tariff BELGIUM 1969 1969 1976 (Benelux) (Benelux) (Benelux) BOLIVIA 1970 1974 modif. up to 1971 BRAZIL 1973 1973 1976 BULGARIA 1968 1972 modif. up to 1974 BURMA 1962 1948 1967 modif. up to 1963 BURUNDI 1970 1975 modify. up to 1974 CAMEROON 1967 1971 (UDEAC) modif. up to 1974 CANADA 1971 1974 modif. up to 1976 up to 1976 CAPE VERDE 1966 * According to D.H.A., a now tariff entered into force in 1975. MTN/TAR/W/5/Rev.1 Page 4 Country Handels-Archiv Brussels Bureau National Tariff CENTRAL AFRICAN 1967 1971 REPUBLIC (UDEAC) modif. -

Signed at Paris on 27 January 1973
No. 13295 (a) UNITED STATES OF AMERICA, DEMOCRATIC REPUBLIC OF VIET-NAM, PROVISIONAL REVOLUTIONARY GOVERNMENT OF THE REPUBLIC OF SOUTH VIET-NAM and REPUBLIC OF VIET-NAM Agreement on ending the war and restoring peace in Viet-Nam. Signed at Paris on 27 January 1973 Authentic texts : English and Vietnamese. (A) UNITED STATES OF AMERICA and DEMOCRATIC REPUBLIC OF VIET-NAM Agreement on ending the war and restoring peace in Viet-Nam. Signed at Paris on 27 January 1973 Authentic texts : English and Vietnamese. Registered by the United States of America on 13 May 1974. (ab) CAMBODIA, DEMOCRATIC REPUBLIC OF VIET-NAM, FRANCE, LAOS, PEOPLE©S REPUBLIC OF CHINA, STATE OF VIET-NAM, UNION OF SOVIET SOCIALIST REPUBLICS, UNITED KINGDOM OF GREAT BRITAIN AND NORTHERN IRELAND and UNITED STATES OF AMERICA Final Declaration of the Geneva Conference on the problem of restoring peace in Indo-China (with (1) declarations by Cambodia, France, Laos, the State of Viet-Nam and the United States of America; (2) the Agreement on the cessation of hostilities in Viet-Nam between the Commander-in-Chief of the People©s Army of Viet-Nam and the Commander-in-Chief of the French Union Forces in Indo-China, signed at Geneva on 20 July 1954 (with maps); (3) the Agreement on the cessa tion of hostilities in Laos between the Commander-in-Chief of the forces of the French Union in Indo-China, on the one hand, and the Commanders-in-Chief of the fighting units of " Pathet- Lao © © and of the People©s Army of Viet-Nam, on the other hand, done at Geneva on 20 July 1954, and (4) the Agreement on the cessation of hostilities in Cambodia between the Commander- in-Chief of the Khmer National Armed Forces, on the one hand, and the Commanders-in-chief of the Khmer Resistance Forces and of the Viet-Namese Military Units, on the other hand, done at Geneva on 20 July 1954). -

Country Term # of Terms Total Years on the Council Presidencies # Of
Country Term # of Total Presidencies # of terms years on Presidencies the Council Elected Members Algeria 3 6 4 2004 - 2005 December 2004 1 1988 - 1989 May 1988, August 1989 2 1968 - 1969 July 1968 1 Angola 2 4 2 2015 – 2016 March 2016 1 2003 - 2004 November 2003 1 Argentina 9 18 15 2013 - 2014 August 2013, October 2014 2 2005 - 2006 January 2005, March 2006 2 1999 - 2000 February 2000 1 1994 - 1995 January 1995 1 1987 - 1988 March 1987, June 1988 2 1971 - 1972 March 1971, July 1972 2 1966 - 1967 January 1967 1 1959 - 1960 May 1959, April 1960 2 1948 - 1949 November 1948, November 1949 2 Australia 5 10 10 2013 - 2014 September 2013, November 2014 2 1985 - 1986 November 1985 1 1973 - 1974 October 1973, December 1974 2 1956 - 1957 June 1956, June 1957 2 1946 - 1947 February 1946, January 1947, December 1947 3 Austria 3 6 4 2009 - 2010 November 2009 1 1991 - 1992 March 1991, May 1992 2 1973 - 1974 November 1973 1 Azerbaijan 1 2 2 2012 - 2013 May 2012, October 2013 2 Bahrain 1 2 1 1998 - 1999 December 1998 1 Bangladesh 2 4 3 2000 - 2001 March 2000, June 2001 2 Country Term # of Total Presidencies # of terms years on Presidencies the Council 1979 - 1980 October 1979 1 Belarus1 1 2 1 1974 - 1975 January 1975 1 Belgium 6 11 11 2019 - 2020 0 2007 - 2008 June 2007, August 2008 2 1991 - 1992 April 1991, June 1992 2 1971 - 1972 April 1971, August 1972 2 1955 - 1956 July 1955, July 1956 2 1947 - 1948 February 1947, January 1948, December 1948 3 Benin 2 4 3 2004 - 2005 February 2005 1 1976 - 1977 March 1976, May 1977 2 Bolivia 3 6 7 2017 - 2018 June