Appendix-I-Water-Constituents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grey Water Reuse for Agricultural Purposes in the Jordan Valley: Household Survey Results in Deir Alla
Water 2012, 4, 580-596; doi:10.3390/w4030580 OPEN ACCESS water ISSN 2073-4441 www.mdpi.com/journal/water Article Grey Water Reuse for Agricultural Purposes in the Jordan Valley: Household Survey Results in Deir Alla Othman A. Al-Mashaqbeh 1,*, Ayoup M. Ghrair 1 and Sharon B. Megdal 2 1 Royal Scientific Society, Knowledge, Amman-al Jubaiha 11941, Jordan; E-Mail: [email protected] 2 Water Resources Research Center, University of Arizona, Tucson, AZ 85721, USA; E-Mail:[email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +962-6-5344701; Fax: +962-6-5344806. Received: 19 June 2012; in revised form: 19 July 2012 / Accepted: 20 July 2012 / Published: 6 August 2012 Abstract: Installation of decentralized grey water treatment systems in small rural communities contributes to a more sustainable water supply. In order to gauge community attitudes about collection and use of grey water, a door-to-door survey in the farming community of Deir Alla, Jordan was conducted by Royal Scientific Society interviewers. Outcomes of a detailed survey, designed specifically for this project, offer insights on people’s views on general water and wastewater issues, as well as their motivation, practices and concerns related to using grey water treatment for a portion of their household wastewater and reuse of the treated grey water for irrigation. A total of 47 respondents from different socio-economic background, aged over 18 years, from this community in the Jordan valley took part in the survey. The level of formal education of the respondents was low, and most of households’ incomes were below the poverty line in Jordan. -

Reuse of Domestic Greywater for the Irrigation of Food Crops
Reuse of Domestic Greywater for the Irrigation of Food Crops Sara Finley Department of Bioresource Engineering McGill University August 2008 Thesis submitted to McGill University in partial fulfillment of the requirements of the Master of Science degree © Sara Finley, 2008 I Abstract As global water resources decline, reuse of domestic greywater for the irrigation of home gardens is quickly becoming widespread in many parts of the world. However, the sanitary implications of reusing greywater to water edible crops remain uncertain. This study examined the benefits and risks associated with domestic greywater reuse for the purposes of vegetable garden irrigation. Untreated (settled only) and treated (settling and slow sand filtration) greywater collected from a family home was analyzed for basic water quality parameters, over a period of eight weeks. During that time, both greywaters were used to irrigate individually potted plots of lettuce, carrots and peppers in a greenhouse. Tap water was used as control. Upon maturity, plants were harvested and the edible portions tested for fecal coliforms and fecal streptococci, common indicators for the presence of pathogenic microorganisms. Heavy metals were not detected in the greywater, but both fecal coliforms and fecal streptococci were present in high levels, averaging 4 x 10 5/100mL and 2 x 10 3/100mL of greywater, respectively. Despite these high counts, no significant difference in contamination levels was observed between crops irrigated with tap water, untreated and treated greywaters. Fecal coliform levels were highest in carrots and fecal streptococcus levels highest on lettuce leaves. However, contamination levels for all crops were low and do not represent a significant health risk. -

Grey Water Systems
Wednesday Water Workshops Grey Water Systems Grey Water Systems • Drought & Mandatory Water Waste Restrictions • Greg Mahoney, City of Davis Chief Building Official • EcoAssistant—Leslie Crenna & Chrissy Backman • Grow Water—Rodger Sargent & Chris Lopez • Nexus eWater—Bob Hitchner • Questions California Drought • September 2: City Council calls Stage 3 water shortage, enacts water restrictions • Water Board Emergency regulations: 28% reduction – June 2015—February 2016 compared to 2013 • June 2015: 32% reduction Mandatory Water Waste Restrictions • Outdoor watering only 3 days a week* – Odd numbered addresses: Tuesday, Thursday and Saturday – Even numbered addresses: Wednesday, Friday and Sunday – No outdoor watering on Monday • No watering between 9am –6pm* – Hose with a shut off nozzle OK – Handheld container OK • No watering during and within 48 hours of rain *properly operating drip and soaker hoses are exempt Mandatory Water Waste Restrictions • No excessive water flow or runoff onto pavement, gutters or ditches from irrigation • No washing off paved surfaces unless necessary for sanitation or safety – Hand held bucket – Hose with shut‐off nozzle – Cleaning machine that recycles water – Low volume/high pressure water broom • Property owners must fix leaks immediately or within 72 hours of notification by the City. • Fountains and water features must have recirculating water Wednesday Water Workshops The City of Davis is hosting a series of water conservation workshops that will be held at 6:30 p.m. on Wednesday evenings at the Veteran’s Memorial Center in the Game Room. Each workshop will feature local subject matter experts. May 6: Rain Water Retention May 20: Lawn Conversion June 10: Irrigation Systems, Controllers & Watering Schedules June 24: Plant Selection & Drought Tolerant Landscaping July 15: Grey Water Systems July 29: Large Scale Impacts of Drought August 5: Taking Care of Trees in a Drought The City of Davis does not recommend, sponsor or otherwise promote any of the businesses that participate in these workshops. -

Residential Greywater Irrigation Systems in California
DRAFT: Residential Greywater Irrigation Systems in California. Greywater Action Residential Greywater Irrigation Systems in California: An Evaluation of Soil and Water Quality, User Satisfaction, and Installation Costs Greywater Action in collaboration with City of Santa Rosa and Ecology Action of Santa Cruz 1 Residential Greywater Irrigation Systems in California: An Evaluation of Soil and Water Quality, User Satisfaction, and Installation Costs November, 2012, revised September 2013 Authors: Laura Allen*1 (Greywater Action) Sherry Bryan (Ecology Action of Santa Cruz) Cleo Woelfle-Erskine (Greywater Action) Contributors: Neeraja Havaligi (PhD Candidate, Akamai University, USA; Climate Change Adaptation Expert at UNDP Bratislava Regional Centre Expert Roster) Susie Murray (Utilities Department, City of Santa Rosa) Greywater Action www.greywateraction.org A project of the Ecology Center 2530 San Pablo Avenue Berkeley, CA 94702 Comments: Please send questions, comments and suggestions to Laura Allen ([email protected]). Copyright: Creative Commons Attribution- Share Alike 3.0 Unported License. 1 *Corresponding author. Email [email protected] Acknowledgments We thank the following people for their technical support, time, and expertise on this study including, Rachel Abramson (City of Santa Rosa), Nik Bertulis (DIG Cooperative), Christina Bertea (Greywater Action), Zachary Burt (UC Berkeley), the Ecology Center, Mike Galloway (Soil Control Laboratory), Richard Harris (EBMUD), Tara Hui (Greywater Action), Natalie Kilmer, Cliff Low (Perry Laboratory), Kara Nelson (UC Berkeley), Walter Norosky (City of Santa Rosa), Ann Northrup (Merritt College Horticulture Department), Stephen Norwick (Sonoma State University), Sharada Prasad (UC Berkeley), Peter Ralph (UC Davis), Isha Ray (UC Berkeley), Kristine Tjung (UC Berkeley), and Gwendolyn von Klan (UC Berkeley). We also thank all the greywater system owners for participating in the study. -

Lesson B1 RESOURCE MANAGEMENT SANITATION
EMW ATER E -LEARNING COURSE PROJECT FUNDED BY THE EUROPEAN UNION LESSON A1: C HARACTERISTIC , A NALYTIC AND SAMPLING OF WASTEWATER Lesson B1 RESOURCE MANAGEMENT SANITATION Authors: Holger Gulyas Deepak Raj Gajurel Ralf Otterpohl Institute of Wastewater Management Hamburg University of Technology Hamburg, Germany Revised by Dr. Yavuz Özoguz data-quest Suchi & Berg GmbH Keywords Anaerobic digestion, Bio-gas, Black water, Brown water, Composting/Vermicomposting, Composting/dehydrating toilet, Ecological sanitation, Grey water, Rottebehaelter, Sorting toilet, Vacuum toilet, Yellow water, EMW ATER E -LEARNING COURSE PROJECT FUNDED BY THE EUROPEAN UNION LESSON A1: C HARACTERISTIC , A NALYTIC AND SAMPLING OF WASTEWATER Table of content 1. Material flows in domestic wastewater....................................................................4 1.1 Different sources..................................................................................................4 1.2 Characteristics of different streams...................................................................4 1.3 Yellow water as fertilizer .....................................................................................6 1.4 Brown water as soil conditioner.........................................................................8 2. Conventional sanitation systems and their limitations..........................................9 3. Conventional decentralised sanitation systems – benefits and limitations.......12 4. Resource Management Sanitation .........................................................................14 -

State of Georgia, Gray Water Recycling System Guidelines
Georgia Gray Water Recycling Systems Guidelines In accordance with Appendix C ‘Gray Water Recycling Systems’ of the 2009 amendments to the 2006 International Plumbing Code 2009 1 Georgia Gray Water Recycling Systems Guidelines PREFACE The Georgia Gray Water Recycling Systems Guidelines are intended to assist all parties involved in the design, construction, inspection and maintenance of gray water recycle systems and to help successfully comply with Appendix C-‘Gray Water Recycling Systems’ of the 2009 Georgia Amendments to the 2006 International Plumbing Code (IPC). The parties mentioned above include owners, building officials, design professionals and contractors. This consensus document is the product of the parties listed below: Guideline Committee Members: Frances Carpenter, Chairperson Bill Guinade Max Rietschier Marvin Richards Bill Morris Tom Carty Ernest U. Earn Bob Freeman David Freedman Philip T. McCreanor, Ph.D Randy Starr Eddie Van Giesen Chris Kumnick Shawn Hatley Ryan Meres Frank Henning Gregory Jeffers Nicole Nichols The Guidelines Committee wishes to take this opportunity to express our sincere appreciation to Chris Kumnick and those individuals who donated their time and effort to the development and production of this document. Disclaimer and Notice: While the information presented in these guidelines is believed to be correct, the parties involved assume no responsibility for its accuracy or for the opinions expressed herein. The material presented in this publication is not considered “Code” and should only be used for reference and guidance in complying with the requirements of Appendix C ‘Gray Water Recycling Systems.’ All gray water recycling systems shall comply with the Georgia State Minimum Standard 2 Plumbing Code (2006 IPC with Georgia Amendments) and all other applicable State Minimum Standard Codes for construction. -

Evolving Roles of Blue, Green, and Gray Water in Agriculture
, Issue 165 December 2018 Evolving Roles of Blue, Green, and Gray Water in Agriculture A publication of the Universities Council on Water Resources with support from Southern Illinois University Carbondale JOURNAL OF CONTEMPORARY WATER RESEARCH & EDUCATION Universities Council on Water Resources 1231 Lincoln Drive, Mail Code 4526 Southern Illinois University Carbondale, IL 62901 Telephone: (618) 536-7571 www.ucowr.org CO-EDITORS Karl W.J. Williard Jackie F. Crim Southern Illinois University Southern Illinois University Carbondale, Illinois 62901 Carbondale, Illinois 62901 [email protected] [email protected] ISSUE EDITORS Paula L.S. Rees Marie-Françoise Hatte Assistant Dean for Diversity Interim Director College of Engineering Water Resources Research Center University of Massachusetts University of Massachusetts Amherst, MA 01003 Amherst, MA 01003 [email protected] [email protected] ASSOCIATE EDITORS Kofi Akamani Natalie Carroll Prem B. Parajuli Policy and Human Dimensions Education Engineering and Modeling Southern Illinois University Purdue University Mississippi State University [email protected] [email protected] [email protected] M.S. Srinivasan Kevin Wagner Jonathan Yoder Hydrology Water Quality and Watershed Management Natural Resource Economics National Institute of Water and Texas A&M University Washington State University Atmospheric Research, New Zealand [email protected] [email protected] [email protected] TECHNICAL EDITORS Elaine Groninger Shelly Williard Southern Illinois University Southern Illinois University Carbondale, Illinois 62901 Carbondale, Illinois 62901 [email protected] [email protected] ISSN 1936-7031 Cover photo: Irrigation, Credit: Doug Parker Back cover photo: Summertime in Snowbird, Credit: Snowbird Inside back cover photo: Aerial Tram, Credit: Snowbird Subscription Information: The Journal of Contemporary Water Research & Education is published by the Universities Council on Water Resources. -

Analysis of Greywater Use for Toilet Flushing in San Francisco
Hatano, Liu 1 Ryan Hatano and Ronald Liu ESM 121: Water Management Professor Sam. Solis June 4, 2015 Analysis of greywater use for toilet flushing in San Francisco University of California, Davis Hatano, Liu 2 Abstract As human population continues to grow rapidly, demand for water quantity and quality is experienced worldwide. Recent technology has explored the idea of utilizing graywater as opposed to the low flush toilets in common households. Our objective is to determine if the cost-benefit-analysis proves that upgrading to toilets utilizing graywater will reduce money and water consumption. We expect our results to demonstrate significant water and cost savings when comparing the low flush toilet to toilets utilizing graywater. Graywater is defined as wastewater created from human-induced activities such as showering, bathing, washing dishes, etc. The interesting aspect of using graywater for toilet flushing is due to it containing a minimal amount of human waste. Toilets water is purely mechanical in nature, as the water physically moves waste through the sewer. As such, this water does not need to be clean. Graywater toilets use water from showering or dish washing for flushing. Currently, low flush toilets use 1.8 gallons of pure water per flush. A typical efficient home could generate about 25,000 gallons of graywater per year. This equates to at least 15,000 flushes per year or 4,250 “free” flushes per person in a household of four. This paper investigates the monetary saving of switching to graywater for the entire city of San Francisco. The water savings of every household switching to a graywater toilet are compared to the costs of implementing such a plan. -

Rainwater Harvesting and Grey Water Reuse (PDF)
Rainwater Harvesting and Grey Water Reuse (CMHC Canada Mortgage and Housing Cor ... Page 1 of 4 Technical series 03-100 Rainwater Harvesting and Grey Water Reuse Introduction Many countries, including some regions in Canada, have a limited amount of water that can be economically delivered to residences as potable water. Limiting factors may be regional, seasonal, remote or urban. They may also relate to the ambient quality of available water and the technology available to treat the water. To help meet water demand, rainwater harvesting and grey water practices are commonly used in several European countries, as well as some others, whereas they are less frequently employed in North America and effectively prohibited by regulation or custom. To better understand current practices in North America and elsewhere, the Canadian Water and Wastewater Association (CWWA), on behalf of Canada Mortgage and Housing Corporation (CMHC), undertook to review practices involving water reuse in residential and other buildings. This included looking at regulations and standards governing non-potable water. Methodology CWWA distributed a questionnaire to all Canadian provincial and territorial drinking water and plumbing regulators, to the same regulators in all U.S. state and federal jurisdictions and to a wide range of CWWA contacts in other countries. The Association received a total of 48 responses: 6 from Canadian jurisdictions; 8 from U.S. jurisdictions; and 34 from other contacts. Responses came from regulatory bodies, water utilities and water districts. CWWA also researched information using Internet; met with several European suppliers of harvesting equipment and town engineers in Belgium, where harvesting is required by state law; and spoke with officials of France’s Ministry of Health. -
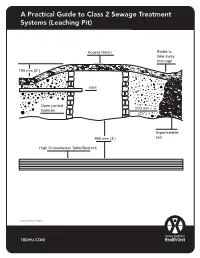
A Practical Guide to Class 2 Sewage Treatment Systems (Leaching Pit)
A Practical guide to class 2 sewage system (leaching pit) A Practical Guide to Class 2 Sewage Treatment Systems (Leaching Pit) Access Hatch Swale to take away drainage 150 mm (6”) Inlet Open jointed 500 mm material (24”) Impermeable 900 mm (3’) soil High Groundwater Table/Bedrock Revised: March 17, 2016 TBDHU.COM Leaching Pits Class 2 Sewage Treatment and Dispersal Systems Leaching pits are used to treat and disperse grey water sewage only. They are not to be used to dispose of toilet water. Grey water sewage comes from plumbing fixtures like sinks, showers or laundry machines. Because they treat and disperse sewage, they must be inspected an approved under the Ontario Building Code by the Thunder Bay District Health Unit. This method of waste water treatment and dispersal is only recommended if there is no pressurized water supply and the daily volume of grey water is small. Under the Ontario Building Code, a leaching pit can only be used if the daily flow of grey water is less than 1000 litres per day. Location Minimum distances must be followed - 15m (50 feet) from any lake, pond, river, stream or any other water course - 15m (50 feet) from any dug well, sand point well, spring or other source of potable water These distances are minimums and may have to be increased if the soil conditions are not ideal. The area should be elevated and well drained. Low laying areas are subject to excessive run off which will overload the pit and saturate the soils. This could result in poor treatment and increases the possibility of ground water contamination. -

Greywater Filtration Systems for a Sustainable Water Culture
Greywater Filtration systems For a sustainable water culture Dr. Ayoup M. Ghrair 20.11.2011- Amman Clean Technology applications in Energy, water and environment workshop Our Research Team DEFINITIONS Greywater is the wastewater collected from household activities except toilet wastewater or except toilet & kitchen wastewater Greywater Treatment Systems: These are systems used to treat domestic greywater in order to be suitable for restricted irrigation of home gardens. (treated to a standard satisfactory for intended reuse). Restricted irrigation: is irrigation of all types of crops with treated greywater except vegetables and plants including edible parts that can get in direct contact with irrigation water wither these parts are eaten raw or cooked. Introduction and back ground: • Jordan is located in an arid to semi arid area and is one of the top water scarce countries in the world. • Grey water represents 50-70 % of the domestic wastewater (grey water is a sustainable source of water ). • Agriculture is the major consumer of water in Jordan, where about 64% of water demand goes for agricultural uses. • Improvement of grey water and utilize it for agricultural application rationally are essentials. Grey water filtration systems can help you save 35% to 40% on your annual water bill, and while saving money, you will also help save the environment and provide a better future for our children. "UNESCO has predicted that by 2020 water shortage will be a serious worldwide problem.” The Grey Water Recycling System is a must for anyone who cares about the environment to replenish the water shortage and reduces environmental pollution as well as waste water production. -

Overview of Greywater Reuse: the Potential of Greywater Systems to Aid Sustainable Water Management
Overview of Greywater Reuse: The Potential of Greywater Systems to Aid Sustainable Water Management Overview of Greywater Reuse: The Potential of Greywater Systems to Aid Sustainable Water Management November 2010 Authors: Lucy Allen Juliet Christian-Smith Meena Palaniappan Pacific Institute 654 13th Street, Preservation Park Oakland, California 94612 www.pacinst.org Phone: 510.251.1600 Facsimile: 510.251.2203 © Copyright 2010. All Rights Reserved ISBN: 1-893790-27-4 ISBN 13: 978-1-893790-29-2 Cover Photo: ©Yanc/Dreamstime.com 1 Overview of Greywater Reuse: The Potential of Greywater Systems to Aid Sustainable Water Management About the Pacific Institute The Pacific Institute is one of the world’s leading independent nonprofits conducting research and education to create a healthier planet and sustainable communities. Based in Oakland, California, we conduct interdisciplinary research and partner with stakeholders to produce solutions that advance environmental protection, economic development, and social equity— in California, nationally, and internationally. We work to change policy and find real-world solutions to problems like water shortages, habitat destruction, global warming, and environmental injustice. Since our founding in 1987, the Pacific Institute has become a locus for independent, innovative thinking that cuts across traditional areas of study, helping us make connections and bring opposing groups together. The result is effective, actionable solutions addressing issues in the fields of freshwater resources, climate change, environmental justice, and globalization. More information about the Institute and our staff, directors, funders, and programs can be found at www.pacinst.org. About the Authors Lucy Allen Lucy Allen is a research associate with the Pacific Institute’s Water Program.