Pathophysiological and Clinical Aspects of Breathing After Stroke 701
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hyperventilation Syndrome
Hyperventilation Syndrome INTRODUCTION ● hyperventilation in the presence of the following should immediately confirm an alternative Hyperventilation syndrome is defined as “a rate of diagnosis: ventilation exceeding metabolic needs and higher than that required to maintain a normal level of plasma CO2”. – cyanosis Physiological hyperventilation can occur in a number of – reduced level of consciousness situations, including life-threatening conditions such as: – reduction in SpO2. ● pulmonary embolism ● diabetic ketoacidosis MANAGEMENT 1,2 ● asthma If ABCD need correction then treat as per medical ● hypovolaemia. guidelines as it is unlikely to be due to hyperventilation syndrome but is more likely to be physiological As a rule, hyperventilation due to emotional stress is hyperventilation secondary to an underlying rare in children, and physical causes are much more pathological process. likely to be responsible for hyperventilation. Maintain a calm approach at all times. Specific presenting features can include: Reassure the patient and try to remove the source of ● acute anxiety the patient’s anxiety, this is particularly important in ● tetany due to calcium imbalance children. ● numbness and tingling of the mouth and lips Coach the patient’s respirations whilst maintaining a calm environment. ● carpopedal spasm ● aching of the muscles of the chest ADDITIONAL INFORMATION ● feeling of light headedness or dizziness. The cause of hyperventilation cannot always be determined with sufficient accuracy (especially in the HISTORY early stages) in the pre hospital environment. Refer to dyspnoea guide Always presume hyperventilation is secondary to hypoxia or other underlying respiratory disorder until proven otherwise. 1,2 ASSESSMENT The resulting hypocapnia will result in respiratory Assess ABCD’s: alkalosis bringing about a decreased level of serum ionised calcium. -

Asphyxia Neonatorum
CLINICAL REVIEW Asphyxia Neonatorum Raul C. Banagale, MD, and Steven M. Donn, MD Ann Arbor, Michigan Various biochemical and structural changes affecting the newborn’s well being develop as a result of perinatal asphyxia. Central nervous system ab normalities are frequent complications with high mortality and morbidity. Cardiac compromise may lead to dysrhythmias and cardiogenic shock. Coagulopathy in the form of disseminated intravascular coagulation or mas sive pulmonary hemorrhage are potentially lethal complications. Necrotizing enterocolitis, acute renal failure, and endocrine problems affecting fluid elec trolyte balance are likely to occur. Even the adrenal glands and pancreas are vulnerable to perinatal oxygen deprivation. The best form of management appears to be anticipation, early identification, and prevention of potential obstetrical-neonatal problems. Every effort should be made to carry out ef fective resuscitation measures on the depressed infant at the time of delivery. erinatal asphyxia produces a wide diversity of in molecules brought into the alveoli inadequately com Pjury in the newborn. Severe birth asphyxia, evi pensate for the uptake by the blood, causing decreases denced by Apgar scores of three or less at one minute, in alveolar oxygen pressure (P02), arterial P02 (Pa02) develops not only in the preterm but also in the term and arterial oxygen saturation. Correspondingly, arte and post-term infant. The knowledge encompassing rial carbon dioxide pressure (PaC02) rises because the the causes, detection, diagnosis, and management of insufficient ventilation cannot expel the volume of the clinical entities resulting from perinatal oxygen carbon dioxide that is added to the alveoli by the pul deprivation has been further enriched by investigators monary capillary blood. -

Prolonged Post-Hyperventilation Apnea in Two Young Adults With
Munemoto et al. BioPsychoSocial Medicine 2013, 7:9 http://www.bpsmedicine.com/content/7/1/9 CASE REPORT Open Access Prolonged post-hyperventilation apnea in two young adults with hyperventilation syndrome Takao Munemoto1,4*, Akinori Masuda2, Nobuatsu Nagai3, Muneki Tanaka4 and Soejima Yuji5 Abstract Background: The prognosis of hyperventilation syndrome (HVS) is generally good. However, it is important to proceed with care when treating HVS because cases of death following hyperventilation have been reported. This paper was done to demonstrate the clinical risk of post-hyperventilation apnea (PHA) in patients with HVS. Case presentation: We treated two patients with HVS who suffered from PHA. The first, a 21-year-old woman, had a maximum duration of PHA of about 3.5 minutes and an oxygen saturation (SpO2) level of 60%. The second patient, a 22-year-old woman, had a maximum duration of PHA of about 3 minutes and an SpO2 level of 66%. Both patients had loss of consciousness and cyanosis. Because there is no widely accepted regimen for treating patients with prolonged PHA related to HVS, we administered artificial ventilation to both patients using a bag mask and both recovered without any after effects. Conclusion: These cases show that some patients with HVS develop prolonged PHA or severe hypoxia, which has been shown to lead to death in some cases. Proper treatment must be given to patients with HVS who develop PHA to protect against this possibility. If prolonged PHA or severe hypoxemia arises, respiratory assistance using a bag mask must be done immediately. Keywords: Post-hyperventilation apnea, PHA, Hyperventilation syndrome, HVS, Hypocapnia, Hypoxemia Background incidence of death, severe hypoxia, or myocardial infarc- Hyperventilation syndrome (HVS) is characterized by tion in association with the paper-bag method [2]. -

Seizures in Childhood Cerebral Malaria
SEIZURES IN CHILDHOOD CEREBRAL MALARIA A thesis submitted to the University of London for the degree of Doctor of Medicine June 2001 Jane Margaret Stewart Crawley MB BS, MRCP A \ Bfii ¥ V ProQuest Number: U642344 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U642344 Published by ProQuest LLC(2015). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT Every year, more than one million children in sub-Saharan Africa die or are disabled as a result of cerebral malaria. Seizures complicate a high proportion of cases, and are associated with an increased risk of death and neurological sequelae. This thesis examines the role of seizures in the pathogenesis of childhood cerebral malaria. The clinical and electrophysiological data presented suggest that seizures may contribute to the pathogenesis of coma in children with cerebral malaria. Approximately one quarter of the patients studied had recovered consciousness within 6 hours of prolonged or multiple seizures, or had seizures with extremely subtle clinical manifestations. EEG recording also demonstrated that electrical seizure activity arose consistently from the posterior temporo-parietal region, a “watershed” area of the brain that is particularly vulnerable to hypoxia. -

Hypercapnia in Hemodialysis (HD)
ISSN: 2692-532X DOI: 10.33552/AUN.2020.01.000508 Annals of Urology & Nephrology Mini Review Copyright © All rights are reserved by David Tovbin Hypercapnia in Hemodialysis (HD) David Tovbin* Department of Nephrology, Emek Medical Center, Israel *Corresponding author: David Tovbin, Department of Nephrology, Emek Medical Received Date: February 04, 2019 Center, Afula, Israel. Published Date: February 14, 2019 Introduction 2 case reports and in our experience with similar patients, BiPAP Acute intra-dialytic exacerbation of hypercapnia in hemodialysis prevented intra-dialytic exacerbation of hypercapnia and possibly (HD) patient has been initially reported 18 years ago [1]. Subsequent respiratory arrest [1,2]. In recent years, new interest was raised similar case was reported few years later [2]. Common features of to HD dialysate bicarbonate concentration. After standardizing to both patients were morbid obesity, a previously stable HD sessions and an acute respiratory infection at time of hypercapnia [1,2]. HD pre-dialysis serum bicarbonate level was recommended as >22 patients with decreased ventilation reserve, due to morbid obesity inflammation malnutrition complex and comorbidities midweek mEq/L [11]. As higher dialysate bicarbonate concentration became with or without obstructive sleep apnea (OSA) and/or obesity more prevalent, a large observation cohort study demonstrated hypoventilation syndrome (OHS) as well as chronic obstructive that high dialysate bicarbonate concentration was associated pulmonary disease (COPD), are at increased risk. COPD is common with worse outcome especially in the more acidotic patients among HD patients but frequently under-diagnosed [3]. Most [12]. However, still not enough attention is paid to HD dialysate COPD patients do well during HD with only mild- moderate pCO2 bicarbonate in the increasing number of patients with impaired increases and slightly decreased pH as compared to non-COPD ventilation, and to their risk of intra-dialytic exacerbation of chronic HD patients [2,4]. -

Basic Life Support Health Care Provider
ELLIS & ASSOCIATES Health Care Provider Basic Life Support MEETS CURRENT CPR & ECC GUIDELINES Ellis & Associates / Safety & Health HEALTH CARE PROVIDER BASIC LIFE SUPPORT - I Ellis & Associates, Inc. P.O. Box 2160, Windermere, FL 34786-2160 www.jellis.com Copyright © 2016 by Ellis & Associates, LLC All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the publisher, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law. For permission requests, write to the publisher, addressed “Attention: Permissions Coordinator,” at the address below. Ellis & Associates P.O. Box 2160, Windermere, FL 34786-2160 Ordering Information: Quantity sales. Special discounts are available on quantity purchases by corporations, associations, trade bookstores and wholesalers. For details, contact the publisher at the address above. Disclaimer: The procedures and protocols presented in this manual and the course are based on the most current recommendations of responsible medical sources, including the International Liaison Committee on Resuscitation (ILCOR) 2015 Guidelines for CPR & ECC. Ellis & Associates, however, make no guarantee as to, and assume no responsibility for, the correctness, sufficiency, or completeness of such recommendations or information. Additional procedures may be required under particular circumstances. Ellis & Associates disclaims all liability for damages of any kind arising from the use of, reference to, reliance on, or performance based on such information. Library of Congress Cataloging-in-Publication Data Not Available at Time of Printing ISBN 978-0-9961108-0-8 Unless otherwise indicated on the Credits Page, all photographs and illustrations are copyright protected by Ellis & Associates. -

Chest Pain and the Hyperventilation Syndrome - Some Aetiological Considerations
Postgrad Med J: first published as 10.1136/pgmj.61.721.957 on 1 November 1985. Downloaded from Postgraduate Medical Journal (1985) 61, 957-961 Mechanism of disease: Update Chest pain and the hyperventilation syndrome - some aetiological considerations Leisa J. Freeman and P.G.F. Nixon Cardiac Department, Charing Cross Hospital (Fulham), Fulham Palace Road, Hammersmith, London W6 8RF, UK. Chest pain is reported in 50-100% ofpatients with the coronary arteriograms. Hyperventilation and hyperventilation syndrome (Lewis, 1953; Yu et al., ischaemic heart disease clearly were not mutually 1959). The association was first recognized by Da exclusive. This is a vital point. It is time for clinicians to Costa (1871) '. .. the affected soldier, got out of accept that dynamic factors associated with hyperven- breath, could not keep up with his comrades, was tilation are commonplace in the clinical syndromes of annoyed by dizzyness and palpitation and with pain in angina pectoris and coronary insufficiency. The his chest ... chest pain was an almost constant production of chest pain in these cases may be better symptom . .. and often it was the first sign of the understood if the direct consequences ofhyperventila- disorder noticed by the patient'. The association of tion on circulatory and myocardial dynamics are hyperventilation and chest pain with extreme effort considered. and disorders of the heart and circulation was ackn- The mechanical work of hyperventilation increases owledged in the names subsequently ascribed to it, the cardiac output by a small amount (up to 1.3 1/min) such as vasomotor ataxia (Colbeck, 1903); soldier's irrespective of the effect of the blood carbon dioxide heart (Mackenzie, 1916 and effort syndrome (Lewis, level and can be accounted for by the increased oxygen copyright. -

Appendix A: Codes Used to Define Principal Diagnosis of COVID-19, Sepsis, Or Respiratory Disease
Appendix A: Codes used to define principal diagnosis of COVID-19, sepsis, or respiratory disease ICD-10 code Description N J21.9 'Acute bronchiolitis, unspecified' 1 J80 'Acute respiratory distress syndrome' 2 J96.01 'Acute respiratory failure with hypoxia' 15 J06.9 'Acute upper respiratory infection, unspecified' 7 U07.1 'COVID-19' 1998 J44.1 'Chronic obstructive pulmonary disease with (acute) exacerbation' 1 R05 'Cough' 1 O99.52 'Diseases of the respiratory system complicating childbirth' 3 O99.512 'Diseases of the respiratory system complicating pregnancy, second trimester' 1 J45.41 'Moderate persistent asthma with (acute) exacerbation' 2 B97.29 'Other coronavirus as the cause of diseases classified elsewhere' 33 J98.8 'Other specified respiratory disorders' 16 A41.89 'Other specified sepsis' 1547 J12.89 'Other viral pneumonia' 222 J12.81 'Pneumonia due to SARS-associated coronavirus' 2 J18.9 'Pneumonia, unspecified organism' 8 R09.2 'Respiratory arrest' 1 J96.91 'Respiratory failure, unspecified with hypoxia' 1 A41.9 'Sepsis, unspecified organism' 155 R06.02 'Shortness of breath' 1 J22 'Unspecified acute lower respiratory infection' 4 J12.9 'Viral pneumonia, unspecified' 6 Appendix B: Average marginal effect by month, main analysis and sensitivity analyses Appendix C: Unadjusted mortality rate over time, by age group Appendix D: Results restricted to COVID-19, sepsis, or respiratory disease Any chronic Adjusted mortality Standardized Average marginal Age, median Male, Mortality, Month N condition, (95% Poisson limits) mortality ratio -

Central Neurogenic Hyperventilation Related to Post-Hypoxic Thalamic Lesion in a Child
Neurology International 2016; volume 8:6428 Central neurogenic normal. An emergency brain magnetic reso- nance imaging (MRI) was performed. Correspondence: Pinar Gençpinar, Department of hyperventilation related Although there was no apparent lesion in the Pediatric Neurology, Tepecik Training and to post-hypoxic thalamic lesion brain stem, bilateral diffuse thalamic, putami- Research Hospital, Izmir, Turkey. in a child nal and globus palllideal lesions were detected Tel.: +90.505.887.9258. on MRI (Figures 1 and 2). Examination E-mail: [email protected] Pinar Gençpinar,1 Kamil Karaali,2 revealed tachypnea (respiratory rate, 42/min), but other findings were normal. Arterial blood Key words: Central neurogenic hyperventilation; enay Haspolat,3 O uz Dursun4 thalamus; tachypnea; children. Ş ğ gases (ABGs) were pH, 7.52; PaCO2, 29 mmHg; 1Department of Pediatric Neurology, and PaO2, 142 mmHg. The chest radiograph, Contributions: PG prepared the manuscript; KK Tepecik Training of Research Hospital, electrocardiogram, and echocardiogram were prepared the figures and edited in this respect; 2 Izmir; Department of Radiology, normal. Laboratory studies disclosed the fol- OD and SH edited this manuscript and made final 3Department of Pediatric Neurology, lowing values: hematocrit, 33.7%, white blood version. 4Department of Pediatric Intensive Care cell count, 10.6×109/L; sodium, 140 mEq/L; Unit, Akdeniz University Hospital, potassium, 3.7 mEq/L; serum urea nitrogen; 6 Conflict of interest: the authors declare no poten- Antalya, Turkey mg/dL; creatinine, 0.21 mg/ dL; and glucose, tial conflict of interest. 110 mg/dL. Liver transaminases levels were normal. Serum lactate level was 1.97 mmol/L Received for publication: 23 January 2016. -
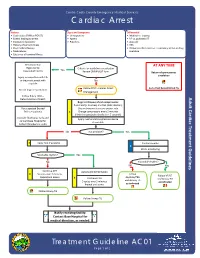
AC01 Page 1 of 2 Effective Jan
Contra Costa County Emergency Medical Services Cardiac Arrest History Signs and Symptoms Differential • Code status (DNR or POLST) • Unresponsive • Medical vs. trauma • Events leading to arrest • Apneic • VF vs. pulseless VT • Estimated downtime • Pulseless • Asystole • History of current illness • PEA • Past medical history • Primary cardiac event vs. respiratory arrest or drug • Medications overdose • Existence of terminal illness Decomposition AT ANY TIME Rigor mortis Criteria for death/no resuscitation Dependent lividity Yes Review DNR/POLST form Return of spontaneous circulation Injury incompatible with life or traumatic arrest with No asystole Follow FP09 ‐ Cardiac Arrest Go to Post Resuscitation TG Do not begin resuscitation Management Follow Policy 1004 – Determination of Death Begin continuous chest compressions Push hard (> 2 inches) and fast (100‐120/min) For suspected Excited Use metronome to ensure proper rate Delirium patients E Change compressors every 2 minutes (Limit changes/pulse checks to < 5 seconds) Consider fluid bolus early and Apply mechanical compression device contact Base Hospital for if available Sodium Bicarbonate order No ALS available? Yes E Apply AED if available Cardiac monitor P EtCO2 monitoring Shockable rhythm? Yes No Shockable rhythm? No Yes Continue CPR Automated defibrillation E 5 cycles over 2 minutes Follow Follow VF/VT Repeat and assess Asystole/PEA E Continue CPR and Airway TG and Airway TG 5 cycles over 2 minutes as indicated Repeat and assess as indicated Follow Airway TG Follow Airway TG Notify receiving facility. Contact Base Hospital for medical direction, as needed. Treatment Guideline AC01 Page 1 of 2 Effective Jan. 2016Effective Jan. 2020 Contra Costa County Emergency Medical Services Cardiac Arrest Pearls • Efforts should be directed at high quality and continuous chest compressions with limited interruptions. -
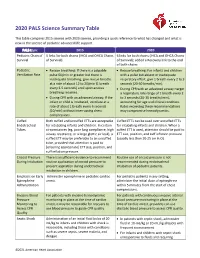
PALS Science Summary Table
2020 PALS Science Summary Table This table compares 2015 science with 2020 science, providing a quick reference to what has changed and what is new in the science of pediatric advanced life support. PALS topic 2015 2020 Pediatric Chain of 5 links for both chains (IHCA and OHCA Chains 6 links for both chains (IHCA and OHCA Chains Survival of Survival) of Survival); added a Recovery link to the end of both chains Pediatric • Rescue breathing: If there is a palpable • Rescue breathing: For infants and children Ventilation Rate pulse 60/min or greater but there is with a pulse but absent or inadequate inadequate breathing, give rescue breaths respiratory effort, give 1 breath every 2 to 3 at a rate of about 12 to 20/min (1 breath seconds (20-30 breaths/min). every 3-5 seconds) until spontaneous • During CPR with an advanced airway: target breathing resumes. a respiratory rate range of 1 breath every 2 • During CPR with an advanced airway: If the to 3 seconds (20-30 breaths/min), infant or child is intubated, ventilate at a accounting for age and clinical condition. rate of about 1 breath every 6 seconds Rates exceeding these recommendations (10/min) without interrupting chest may compromise hemodynamics. compressions. Cuffed Both cuffed and uncuffed ETTs are acceptable Cuffed ETTs can be used over uncuffed ETTs Endotracheal for intubating infants and children. In certain for intubating infants and children. When a Tubes circumstances (eg, poor lung compliance, high cuffed ETT is used, attention should be paid to airway resistance, or a large glottic air leak), a ETT size, position, and cuff inflation pressure cuffed ETT may be preferable to an uncuffed (usually less than 20-25 cm H2O). -

Current Clinical Approach to Patients with Disorders of Consciousness
CURRENTREVIEW CLINICAL APPROA CARTICLEH TO PATIENTS WITH DISORDERS OF CONSCIOUSNESS Current clinical approach to patients with disorders of consciousness ROBSON LUIS OLIVEIRA DE AMORIM1, MARCIA MITIE NAGUMO2*, WELLINGSON SILVA PAIVA3, ALMIR FERREIRA DE ANDRADE3, MANOEL JACOBSEN TEIXEIRA4 1PhD – Assistant Physician of the Neurosurgical Emergency Unit, Division of Neurosurgery, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil 2Nurse – MSc Student at the Neurosurgical Emergency Unit, Division of Neurosurgery, Hospital das Clínicas, FMUSP, São Paulo, SP, Brazil 3Habilitation (BR: Livre-docência) – Professor of the Neurosurgical Emergency Unit, Division of Neurosurgery, Hospital das Clínicas, FMUSP, São Paulo, SP, Brazil 4Habilitation (BR: Livre-docência) – Full Professor of the Division of Neurosurgery, Hospital das Clínicas, FMUSP, São Paulo, SP, Brazil SUMMARY Study conducted at Hospital das Clínicas, In clinical practice, hospital admission of patients with altered level of conscious- Faculdade de Medicina, Universidade de ness, sleepy or in a non-responsive state is extremely common. This clinical con- São Paulo (FMUSP), São Paulo, SP, Brazil dition requires an effective investigation and early treatment. Performing a fo- Article received: 1/28/2015 cused and objective evaluation is critical, with quality history taking and Accepted for publication: 5/4/2015 physical examination capable to locate the lesion and define conducts. Imaging *Correspondence: and laboratory exams have played an increasingly important role in supporting Address: Av. Dr. Enéas de Carvalho Aguiar, 255, Cerqueira César clinical research. In this review, the main types of changes in consciousness are São Paulo, SP – Brazil discussed as well as the essential points that should be evaluated in the clinical Postal code: 05403-000 [email protected] management of these patients.