Indometacin (Indometacinum)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PACKAGE LEAFLET:INFORMATION for the USER Indocid Suppositories 100 Mg Indometacin
PACKAGE LEAFLET:INFORMATION FOR THE USER Indocid Suppositories 100 mg Indometacin Read all of this leaflet carefully before you start using this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or pharmacist • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. In this leaflet: 1. What Indocid Suppositories are and what they are used for 2. Before you use Indocid Suppositories 3. How to use Indocid Suppositories 4. Possible side effects 5, How to store Indocid Suppositories 6. Contents of the pack and other information 1. What Indocid Suppositories are and what they are used for Indocid Suppositories contain the active ingredient indometacin. This belongs to a group of medicines known as ‘non-steroidal anti-inflammatory agents’ or NSAlDs. These work by reducing the body’s ability to produce Inflammation, which may cause pain and discomfort. Your doctor has prescribed Indocid for you because you are suffering from one of the following: • Rheumatoid arthritis (disease mainly of the joints) • Osteoarthritis (disease of the joints) • Ankylosing spondylitis (a form of arthritis which mainly affects the back) • Pain, inflammation, and swelling following orthopaedic surgery. • Musculoskeletal disorders (muscles and bone disorders). • Period pain. • Low back pain. • Disease of the hip joint. -

Celecoxib, Celebrex, Celebra, Onsenal IUPAC- Name
Celecoxib Medical name: Celecoxib, Celebrex, Celebra, Onsenal IUPAC- name: 4-[5-(4-Methylphenyl)-3- (trifluoromethyl) pyrazol- 1-yl]benzenesulfonamide Formula: C17H14F3N3O2S (molar mass: 381,373 g/mol) Celecoxib, also known as Celebrex, is a non-steroidal anti-inflammatory drug (NSAID). It is used as long-term treatment of physical illnesses like osteoarthritis, chronic polyarthritis and Morbus Bechterew. The drug was developed by the US- American pharmacologist Philip Needleman and fielded by a pharmaceutical company called Pfizer. History: As vice president and manager of all chemical actions of the pharmaceutical and biotechnological company Pharmacia, Philip Needleman supervised the study of the experimental research of the enzyme Cyclooxygenase 2 (COX-2). The enzyme already was discovered by the Birmingham Young University in 1991. Its function is transmitting pain signals to the brain. Needleman’s ambition was to find a substance which is capable to inhibit the enzyme. On December, 31 in 1998 the research study lead to the NSAID drug Celecoxib. Rofecoxib, a similar drug which is able to inhibit COX-2 as well, has been taken off the market due to the fact that the drug caused strokes and heart attacks. As a consequence, an excessive demand for Celecoxib arose, despite of analogical adverse effects. Primary, the drug was created as a pain reliever for a long-term treatment with improved effects to the gastrointestinal passage compared to other NSAIDs. It is effective likewise ibuprofen and paracetamol. Celecoxib supposedly is “better in protecting the stomach from serious complications than other drugs”. The company Pharmacia and the concern Pfizer based their advertisement for Celecoxib on this statement. -

B96i. Nsaids 2.1 Approximately 2 Million Prescription Items (13% of All NSAID Items) Per Year in Primary Care in England
Community Interest Company Non-steroidal anti-inflammatory drugs (NSAIDs) Over £70 million is spent annually on all non-steroidal anti-inflammatory drugs (NSAIDs), including cyclo-oxygenase II (COX-2) inhibitors, in England (ePACT Nov 14 - Jan 15). QIPP projects in this area focus on reducing prescribing of NSAIDs for cost and safety reasons, by adhering to current guidance. Recommendations • Regularly review the appropriateness of NSAID prescribing, especially in people who are at higher risk of both gastrointestinal (GI) and cardiovascular (CV) and renal morbidity and mortality, e.g. older people.1 Consider switching to a lower risk NSAID where appropriate. • Advise patients to exercise as a core treatment for osteoarthritis, to improve muscle strength and general aerobic fitness.2 • Consider paracetamol and/or topical NSAIDs (according to local formulary) before oral NSAIDs, COX- 2 inhibitors or opioids.2 • Choose the NSAID with the lowest CV, renal and/or GI risk, depending upon the individual patient’s risk factors.2 • Do not prescribe NSAIDs where contraindicated (CI). Only prescribe NSAIDs in patients at risk of renal impairment/failure (particularly the elderly) where unavoidable.3 • If an NSAID is needed then prescribe the lowest dose for the shortest duration,1-3 e.g. ibuprofen (≤1200mg daily in divided doses) or naproxen (≤1000mg daily in divided doses).1,3 These are associated with a lower CV risk than other NSAIDs.1,3 • COX-2 inhibitors, diclofenac (150mg daily) and ibuprofen (2.4g daily) are associated with an increased risk of thrombotic events. The increased risk for diclofenac is similar to that of licensed doses of etoricoxib.3 • Diclofenac has been associated with increased risk of recurrent myocardial infarction (MI) or death, from the beginning of treatment.4 • Co-prescribe a proton pump inhibitor (PPI) with lowest acquisition cost for gastroprotection in patients with a high risk of GI bleeds in: » Patients ≥65 years2 » Long term NSAID use, e.g. -

Indomethacin in Pregnancy: Applications and Safety
Original Article 175 Indomethacin in Pregnancy: Applications and Safety Gael Abou-Ghannam, M.D. 1 Ihab M. Usta, M.D. 1 Anwar H. Nassar, M.D. 1 1 Department of Obstetrics and Gynecology, American University of Address for correspondence and reprint requests Anwar H. Nassar, Beirut Medical Center, Hamra, Beirut, Lebanon M.D., American University of Beirut Medical Center, P.O. Box 113-6044/B36, Hamra 110 32090, Beirut, Lebanon (e-mail: [email protected]). Am J Perinatol 2012;29:175–186. Abstract Preterm labor (PTL) is a major cause of neonatal morbidity and mortality worldwide. Among the available tocolytics, indomethacin, a prostaglandin synthetase inhibitor, has been in use since the 1970s. Recent studies have suggested that prostaglandin synthetase inhibitors are superior to other tocolytics in delaying delivery for 48 hours and 7 days. However, increased neonatal complications including oligohydramnios, Keywords renal failure, necrotizing enterocolitis, intraventricular hemorrhage, and closure of the ► indomethacin patent ductus arteriosus have been reported with the use of indomethacin. Indometh- ► tocolysis acin has been also used in women with short cervices as well as in those with idiopathic ► preterm labor polyhydramnios. This article describes the mechanism of action of indomethacin and its ► short cervix clinical applications as a tocolytic agent in women with PTL and cerclage and its use in ► polyhydramnios the context of polyhydramnios. The fetal and neonatal side effects of this drug are also ► fetal side effects summarized and guidelines for its use are proposed. Preterm labor (PTL) is a major cause of neonatal morbidity in women with PTL and cerclage and its use in the context of and mortality worldwide.1 Care of premature infants has polyhydramnios. -

Comparison of Pharmacokinetics and Efficacy of Oral and Injectable Medicine Outline
Comparison of pharmacokinetics and efficacy of oral and injectable medicine Outline • Background • Results – Antibiotics – Non steroidal anti-inflammatory drugs (NSAIDs) – Vitamins • Conclusions and recommendations Outline • Background • Results – Antibiotics – Non steroidal anti-inflammatory drugs (NSAIDs) – Vitamins • Conclusions and recommendations Injections given with sterile and reused South America (lower mortality) equipment worldwide Central Europe South America (higher mortality) West Africa Injections given with non-sterile equipment East and Southern Africa Injections given with sterile equipment South East Asia Regions China and Pacific Eastern Europe and Central Asia South Asia Middle East Crescent - 2.0 4.0 6.0 8.0 10.0 12.0 Number of injections per person and per year Injections: A dangerous engine of disease • Hepatitis B – Highly infectious virus – Highest number of infections (21 million annually) – 32% of HBV infections • Hepatitis C – More than 2 million infections each year – More than 40% of HCV infections • HIV – More than 260 000 infections – Approximately 5% of HIV infections Reported common conditions leading to injection prescription • Infections • Asthma – Fever • Other – Upper Respiratory – Malaise Infection/ Ear Infection – Fatigue – Pneumonia – Old Age – Tonsillitis – Pelvic Inflammatory Disease – Skin Infections – Diarrhea – Urinary tract infection Simonsen et al. WHO 1999 Reported injectable medicines commonly used • Antibiotics • Anti-inflammatory agents / Analgesics • Vitamins Simonsen et al. WHO 1999 -

The Acceleration of Articular Cartilage Degeneration in Osteoarthritis by Nonsteroidal Anti-Inflammatory Drugs Ross A
WONDER WHY? THE ACCELERATION OF ARTICULAR CARTILAGE DEGENERATION IN OSTEOARTHRITIS WONDER WHY? The Acceleration of Articular Cartilage Degeneration in Osteoarthritis by Nonsteroidal Anti-inflammatory Drugs Ross A. Hauser, MD A B STRA C T introduction over the past forty years is one of the main causes of the rapid rise in the need for hip and knee Nonsteroidal anti-inflammatory drugs (NSAIDs) are replacements, both now and in the future. among the most commonly used drugs in the world for the treatment of osteoarthritis (OA) symptoms, and are While it is admirable for the various consensus and taken by 20-30% of elderly people in developed countries. rheumatology organizations to educate doctors and Because of the potential for significant side effects of the lay public about the necessity to limit NSAID use in these medications on the liver, stomach, gastrointestinal OA, the author recommends that the following warning tract and heart, including death, treatment guidelines label be on each NSAID bottle: advise against their long term use to treat OA. One of the best documented but lesser known long-term side effects The use of this nonsteroidal anti-inflammatory of NSAIDs is their negative impact on articular cartilage. medication has been shown in scientific studies to accelerate the articular cartilage breakdown In the normal joint, there is a balance between the in osteoarthritis. Use of this product poses a continuous process of cartilage matrix degradation and significant risk in accelerating osteoarthritis joint repair. In OA, there is a disruption of the homeostatic breakdown. Anyone using this product for the pain state and the catabolic (breakdown) processes of of osteoarthritis should be under a doctor’s care and chondrocytes. -

Inflammatory Drugs (Nsaids) for People with Or at Risk of COVID-19
Evidence review Acute use of non-steroidal anti- inflammatory drugs (NSAIDs) for people with or at risk of COVID-19 Publication date: April 2020 This evidence review sets out the best available evidence on acute use of non- steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. It should be read in conjunction with the evidence summary, which gives the key messages. Evidence review commissioned by NHS England Disclaimer The content of this evidence review was up-to-date on 24 March 2020. See summaries of product characteristics (SPCs), British national formulary (BNF) or the MHRA or NICE websites for up-to-date information. For details on the date the searches for evidence were conducted see the search strategy. Copyright © NICE 2020. All rights reserved. Subject to Notice of rights. ISBN: 978-1-4731-3763-9 Contents Contents ...................................................................................................... 1 Background ................................................................................................. 2 Intervention .................................................................................................. 2 Clinical problem ........................................................................................... 3 Objective ...................................................................................................... 3 Methodology ................................................................................................ 4 Summary of included studies -

Non-Steroidal Anti-Inflammatory Drug Use and the Risk of Acute
Drugs - Real World Outcomes (2017) 4:127–137 DOI 10.1007/s40801-017-0113-x ORIGINAL RESEARCH ARTICLE Non-Steroidal Anti-Inflammatory Drug Use and the Risk of Acute Myocardial Infarction in the General German Population: A Nested Case–Control Study 1,2 1 1 Kathrin Tho¨ne • Bianca Kollhorst • Tania Schink Published online: 4 July 2017 Ó The Author(s) 2017. This article is an open access publication Abstract sampling. Multivariable conditional logistic regression was Introduction Use of non-steroidal anti-inflammatory drugs applied to estimate odds ratios (ORs) and 95% confidence (NSAIDs) has been associated with an increased relative intervals (CIs). Duration of NSAID use was calculated by risk of acute myocardial infarction (AMI), but the label the cumulative amount of dispensed defined daily doses warnings refer particularly to patients with cardiovascular (DDDs), and stratified analyses were conducted for risk factors. The magnitude of relative AMI risk for potential effect modifiers. patients with and without cardiovascular risk factors varies Results Overall, 17,236 AMI cases were matched to between studies depending on the drugs and doses studied. 1,714,006 controls. Elevated relative AMI risks were seen Objectives The aim of our study was to estimate popula- for current users of fixed combinations of diclofenac with tion-based relative AMI risks for individual and widely misoprostol (OR 1.76, 95% CI 1.26–2.45), indometacin used NSAIDs, for a cumulative amount of NSAID use, and (1.69, 1.22–2.35), ibuprofen (1.54, 1.43–1.65), etoricoxib for patients with and without a prior history of cardiovas- (1.52, 1.24–1.87), and diclofenac (1.43, 1.34–1.52) com- cular risk factors. -

COX-2 Selective Non-Steroidal Anti-Inflammatory Drugs For
Health Technology Assessment Health Technology Health Technology Assessment 2008; Vol. 12: No. 11 2008; 12: No. 11 Vol. selective non-steroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis COX-2 Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation Feedback The HTA Programme and the authors would like to know Y-F Chen, P Jobanputra, P Barton, S Bryan, your views about this report. The Correspondence Page on the HTA website A Fry-Smith, G Harris and RS Taylor (http://www.hta.ac.uk) is a convenient way to publish your comments. If you prefer, you can send your comments to the address below, telling us whether you would like us to transfer them to the website. We look forward to hearing from you. April 2008 The National Coordinating Centre for Health Technology Assessment, Mailpoint 728, Boldrewood, Health Technology Assessment University of Southampton, NHS R&D HTA Programme Southampton, SO16 7PX, UK. HTA Fax: +44 (0) 23 8059 5639 Email: [email protected] www.hta.ac.uk http://www.hta.ac.uk ISSN 1366-5278 HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. -

Treatment of Acute Gout: a Systematic Review
Seminars in Arthritis and Rheumatism 44 (2014) 31–38 Contents lists available at ScienceDirect Seminars in Arthritis and Rheumatism journal homepage: www.elsevier.com/locate/semarthrit Treatment of acute gout: A systematic review Puja P. Khanna, MD, MPHa,n,1, Heather S. Gladue, DOc, Manjit K. Singh, MDd, John D. FitzGerald, MD, PhDe,2, Sangmee Bae, MDe, Shraddha Prakash, MDe, Marian Kaldas, MDe, Maneesh Gogia, MDe, Veronica Berrocal, PhDa, Whitney Townsend, MLISa,b, Robert Terkeltaub, MDf,3, Dinesh Khanna, MD, MSa,4 a Division of Rheumatology, University of Michigan, 300 North Ingalls, 7D13, Ann Arbor, MI 48109-5422 b Taubman Health Science Library, University of Michigan, 300 North Ingalls, 7D13, Ann Arbor, MI 48109-5422 c Emory University, Atlanta, GA d Rochester General Health System, Rochester, NY e David Geffen School of Medicine, UCLA, Los Angeles, CA f VAMC/UCSD, La Jolla, CA article info abstract Objective: Acute gout is traditionally treated with NSAIDs, corticosteroids, and colchicine; however, subjects have multiple comorbidities that limit the use of some conventional therapies. We systemati- Keywords: Systematic review cally reviewed the published data on the pharmacologic and non-pharmacologic agents used for the Acute gout treatment of acute gouty arthritis. Methods: A systematic search was performed using PubMed and Cochrane database through May 2013. We included only randomized controlled trials (RCTs) that included NSAIDs, corticosteroids, colchicine, adrenocorticotropic hormone (ACTH), interleukin-1 (IL-1) inhibitors, topical ice, or herbal supplements. Results: Thirty articles were selected for systematic review. The results show that NSAIDs and COX-2 inhibitors are effective agents for the treatment of acute gout attacks. -
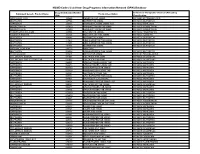
NSAID Codes Used from Drug Programs Information Network (DPIN) Database
NSAID Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Number Anatomical Therapeutic Chemical (ATC) Drug Equivalent Generic Product Name Product Description (DIN) Classification MEFENAMIC ACID 155225 PONSTAN CAP 250MG M01AG01 MEFENAMIC ACID IBUPROFEN 327794 MOTRIN TAB 300MG M01AE01 IBUPROFEN NAPROXEN 335193 NAPROSYN 250MG 250MG TAB M01AE02 NAPROXEN INDOMETHACIN 337420 NOVO-METHACIN CAP 25MG M01AB01 INDOMETACIN INDOMETHACIN 337439 NOVO-METHACIN CAP 50MG M01AB01 INDOMETACIN FENOPROFEN CALCIUM 345504 NALFON TAB 600MG M01AE04 FENOPROFEN TOLMETIN SODIUM 364126 TOLECTIN 200 TAB 200MG M01AB03 TOLMETIN IBUPROFEN 364142 MOTRIN TAB 400MG M01AE01 IBUPROFEN IBUPROFEN 441643 APO IBUPROFEN TAB 200MG M01AE01 IBUPROFEN IBUPROFEN 441651 APO IBUPROFEN TAB 300MG M01AE01 IBUPROFEN IBUPROFEN 484911 MOTRIN TAB 600MG M01AE01 IBUPROFEN NAPROXEN SODIUM 491772 ANAPROX IBUPROFEN 506052 APO-IBUPROFEN TAB 400MG M01AE01 IBUPROFEN PENICILLAMINE 511641 DEPEN TAB 250MG M01CC01 PENICILLAMINE DICLOFENAC SODIUM 514004 VOLTAREN TAB 25MG M01AB05 DICLOFENAC DICLOFENAC SOD SR 75MG TAB 514012 VOLTAREN TAB 50MG M01AB05 DICLOFENAC NAPROXEN 522651 APO NAPROXEN 250MG TAB M01AE02 NAPROXEN NAPROXEN 522678 APO NAPROXEN 125MG TAB M01AE02 NAPROXEN NAPROXEN 565350 NOVO-NAPROX TAB 250MG M01AE02 NAPROXEN NAPROXEN 565369 NOVO-NAPROX 125MG TAB M01AE02 NAPROXEN NAPROXEN 583367 NAPROSYN 375MG TAB M01AE02 NAPROXEN IBUPROFEN 585114 APO IBUPROFEN TAB 600MG M01AE01 IBUPROFEN NAPROXEN 587923 NAPROSYN SUS 25MG/ML M01AE02 NAPROXEN NAPROXEN 589861 NOVO-NAPROX TAB -

Indometacin 25Mg and 50Mg Capsules
- speech disorder, fits or seizures, worsening of By reporting side effects you can help provide more epilepsy, disorder of the nerves causing tingling information on the safety of this medicine. and numbness, pins and needles - uncontrolled movements, worsening of 5 How to store Indometacin capsules parkinsonism (tremor, stiffness and shuffling). Keep out of the sight and reach of children. • Heart: Store below 25°C in a dry place. Protect from light. - fluid retention causing swelling, chest pain, heart Do not take Indometacin capsules after the expiry Indometacin 25mg failure (symptoms include shortness of breath, date which is stated on the label/carton/bottle. tiredness, increased heart rate, flushing, swelling - Medicines should not be disposed of via wastewater and 50mg capsules especially of the ankles) or household waste. Ask your pharmacist how to - high and low blood pressure (symptoms include dispose of medicines no longer required. These dizziness, fainting, light-headedness, nausea, measures will help to protect the environment. heart attack) Read all of this leaflet carefully before 2 What you need to know before you take - racing heart beat, irregular heart beat, 6 Contents of the pack and other you start taking this medicine because it Indometacin capsules palpitations. Do not take Indometacin capsules if you: • Blood vessels: information contains important information for you. • Keep this leaflet. You may need to read it • are in the last three months of pregnancy or - flushing. What Indometacin capsules contain while you are breast-feeding (see “Pregnancy • Breathing: • The active substance (the ingredient that makes the again. and breast-feeding”) - increase in the number of white blood cells in the medicine work) is indometacin PhEur.