Nitrilotriacetic Acid)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toxicological Profile for Formaldehyde
TOXICOLOGICAL PROFILE FOR FORMALDEHYDE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry July 1999 FORMALDEHYDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. FORMALDEHYDE iii UPDATE STATEMENT Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 FORMALDEHYDE vii QUICK REFERENCE FOR HEALTH CARE PROVIDERS Toxicological Profiles are a unique compilation of toxicological information on a given hazardous substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation of available toxicologic and epidemiologic information on a substance. Health care providers treating patients potentially exposed to hazardous substances will find the following information helpful for fast answers to often-asked questions. Primary Chapters/Sections of Interest Chapter 1: Public Health Statement: The Public Health Statement can be a useful tool for educating patients about possible exposure to a hazardous substance. It explains a substance’s relevant toxicologic properties in a nontechnical, question-and-answer format, and it includes a review of the general health effects observed following exposure. Chapter 2: Health Effects: Specific health effects of a given hazardous compound are reported by route of exposure, by type of health effect (death, systemic, immunologic, reproductive), and by length of exposure (acute, intermediate, and chronic). -

Wo 2008/127291 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 23 October 2008 (23.10.2008) WO 2008/127291 A2 (51) International Patent Classification: Jeffrey, J. [US/US]; 106 Glenview Drive, Los Alamos, GOlN 33/53 (2006.01) GOlN 33/68 (2006.01) NM 87544 (US). HARRIS, Michael, N. [US/US]; 295 GOlN 21/76 (2006.01) GOlN 23/223 (2006.01) Kilby Avenue, Los Alamos, NM 87544 (US). BURRELL, Anthony, K. [NZ/US]; 2431 Canyon Glen, Los Alamos, (21) International Application Number: NM 87544 (US). PCT/US2007/021888 (74) Agents: COTTRELL, Bruce, H. et al.; Los Alamos (22) International Filing Date: 10 October 2007 (10.10.2007) National Laboratory, LGTP, MS A187, Los Alamos, NM 87545 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, (30) Priority Data: CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, 60/850,594 10 October 2006 (10.10.2006) US ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, (71) Applicants (for all designated States except US): LOS LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, ALAMOS NATIONAL SECURITY,LLC [US/US]; Los MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, Alamos National Laboratory, Lc/ip, Ms A187, Los Alamos, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, NM 87545 (US). -

Nitrilotriacetic Acid and Its Salts
NITRILOTRICETIC ACID AND ITS SALTS 1. Chemical and Physical Data 1.1 Synonyms Nitrilotriacetic acid (NTA) Chem. Abstr. Services Reg. No.: 139-13-9 (Replaced CAS Reg. Nos 2627-44-1, 2627-45-2 and 80751-51-5) Chem. Abstr. Name: Glycine, N,N-bis( carbxymethyl)- ¡UP AC Systematic Name: Nitriotriacetic acid Synonym: Nitrio-2,2' ,2" -triacetic acid; triglycine; triglycollamic acid; a,a' , a" -trIe- thylaminetricarboxylic acid Nitrilotriacetic acid, sodium salt (unspecified) Chem. Abstr. Services Reg. No.: 1002-84-9 Chem. Abstr. Name: Glycine, N,N-bis(carbxymethyl)-, sodium salt ¡UPAC Systematic Name: Nitriotriacetic acid, sodium salt Synonym: Sodium aminotriacetate; sodium nitrioacetate; sodium nitrilotriacetate; sodium NTA Nitrilotriacetic acid, monosodium salt (NTA, monosodium salt) Chem. Abstr. Services Reg. No: 18994-66-6 Chem. Abstr. Name: Glycine, N,N-bis( carbxymethyl)-, monosodium salt ¡UP AC Systematic Name: Sodium dihydrogen nitriotriacetate Synonym: Monosodium nitrioacetate; monosodium nitriotriacetate; NaNT A Nitrilotriacetic acid, disodium salt (NTA, disodium salt) Chem. Abstr. Services Reg. No.: 15467-20-6 Chem. Abstr. Name: Glycine, N,N-bis( carbxyethyl)-, disodium salt ¡UP AC Systematic Name: Disoium hydrogen nitriotriacetate Synonym: Disodium nitriotriacetate; Na2NTA Nitrilotriacetic acid, disodium salt, monohydrate (NT A disodium salt, monohydrate) Chem. Abstr. Services Reg. No.: 23255-03-0 Chem. Abstr. Name: Glycine, N,N-bis(carbxymethyl)-, disodium salt, monohydrate IUP AC Systematic Name: Disodium hydrogen nitriotriacetate, monohydrate Synonym: Acetic acid, nitriotri-, disoium salt, monohydrate; disodium nitriotria- cetic acid monohydrate; Na2NTA.H20 -181- 182 lAC MONOGRAHS VOLUME 48 Nitrilotriacetic acid, trisodium salt (NTA, trisodium salt) Chem. Abstr. Services Reg. No.: 50-31-3 (Replaced CAS Reg. No. 37291-81-9) Chem. -

Hydrogen Cyanide and Cyanides: Human Health Aspects
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 61 HYDROGEN CYANIDE AND CYANIDES: HUMAN HEALTH ASPECTS Please note that the layout and pagination of this pdf file are not identical to the version in press First draft prepared by Prof. Fina Petrova Simeonova, Consultant, National Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria; and Dr Lawrence Fishbein, Fairfax, Virginia, USA Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2004 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

An-Najah National University Faculty of Graduate Studies
An-Najah National University Faculty of Graduate Studies Spectrophotometric determination of some metal ions via complex formation with carboxylated tris(2-aminoethyl)amine chelating agent By Julnar Adnan Ibrahim Masharqah Supervisor Dr. Ibrahim Abu Shqair Co- Supervisor Dr. Ziad Shakhsher This Thesis is Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Chemistry, Faculty of Graduate Studies, An- Najah National University, Nablus- Palestine. 2014 iii Dedication I dedicate this thesis to all who stood by me and supported my steps in any way and means… My greatest thanks and gratitude to Allah first of all, then to my family, specially my father and mother for their endless encouragement and advice, my sisters and brother … To my uncles, aunts, grandmothers and grandfathers, who encouraged me to go ahead and never give up... To my companions who bear me through all the difficulties I went through, who always push me forward, … To all of you and to those whom I didn't mention, my gratitude and appreciation… iv Acknowledgments At the threshold between graduation and the beginning of a new step in our lives, and before I go on, it is my honor to give the utmost of gratitude and appreciation to those who carried the holiest message in life…. Those who paved the ways of learning and knowledge in front of us… Special thanks to Dr. Ibrahim Abu Shqair and Dr. Ziad Shakhsher, for their efforts that had their prints on finishing and producing this work in its final shape. To those who helped me and facilitated the ways, who inspired me with thoughts and knowledge. -
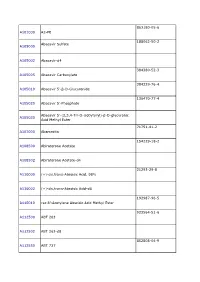
TRC Price List 2010
863180-05-6 A101000 A2-PE 188062-50-2 Abacavir Sulfate A105000 A105002 Abacavir-d4 384380-52-3 A105005 Abacavir Carboxylate 384329-76-4 A105010 Abacavir 5’-β-D-Glucuronide 136470-77-4 A105020 Abacavir 5’-Phosphate Abacavir 5’-(2,3,4-Tri-O-isobytyryl)-β-D-glucuronic A105030 Acid Methyl Ester 71751-41-2 A107000 Abamectin 154229-18-2 A108500 Abiraterone Acetate A108502 Abiraterone Acetate-d4 21293-29-8 A110000 (+)-cis,trans-Abscisic Acid, 98% A110002 (+)-cis,trans-Abscisic Acid-d6 192987-96-5 A110010 rac 8’-Acetylene Abscisic Acid Methyl Ester 923564-51-6 A112500 ABT 263 A112502 ABT 263-d8 852808-04-9 A112550 ABT 737 A112552 ABT 737-d8 912444-00-9 A112580 ABT 888 447407-36-5 A115600 AC 42 77337-73-6 A120000 Acamprosate Calcium A120002 Acamprosate-d12 Calcium Trihydrate A120003 Acamprosate-d6 Calcium Trihydrate 56180-94-0 A123500 Acarbose A123502 Acarbose-d4 117065-98-2 A123505 Acarbose Tridecaacetate 34381-68-5 A123800 Acebutolol Hydrochloride Acebutolol 37517-30-9 A123801 Discontinued. -

RISK ASSESSMENT Trisodium Nitrilotriacetate
R307_hh_env_0808 Draft of 20.08.2008 RISK ASSESSMENT Trisodium Nitrilotriacetate CAS-No.: 5064-31-3 EINECS-No.: 225-768-6 Draft of 20.08.2008 1 R307_0808_hh_env.doc Draft of 11.03.2008 Information on the rapporteur Contact point: Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA) Chemikalien, Anmeldung und Zulassung Federal Institute for Occupational Safety and Health Division for Chemicals and Biocides Regulation Friedrich-Henkel-Weg 1-25 44149 Dortmund fax: +49-(0)231-9071-2679 e-mail: [email protected] The first draft of the Human Health Section of the Comprehensive Risk Assessment Report Trisodium Nitrilotriacetate (NTA), a substance chosen from the EU 3rd Priority List in 1995, which is distributed for the preliminary written procedure was distributed to MS for preliminary comments in October 2006. This document is the revised draft of the Human Health Section of the RAR of NTA which is intended to be discussed in-depth at the TC NES II´07. R307_0808_hh_env.doc Draft of 11.03.2008 Contents 0 OVERALL CONCLUSIONS/RESULTS OF THE RISK ASSESSMENT 4 1 GENERAL SUBSTANCE INFORMATION 7 2 GENERAL INFORMATION ON EXPOSURE 11 2.1 Production, Import and Export 11 2.2 Processing / Application (Categories of use, Amounts) 11 2.3 Use Pattern 13 3 ENVIRONMENT 14 3.1 Environmental exposure 14 3.1.1 General discussion 14 3.1.2 Aquatic compartment 24 3.1.3 Atmosphere 36 3.1.4 Terrestrial compartment 37 3.1.5 Non compartment specific exposure relevant to the food chain 37 3.2 Effects assessment: Hazard identification and Dose (concentration) - -
Inorganic Hydrogen Cyanide Listing Background Document for the Inorganic Chemical Listing Determination
INORGANIC HYDROGEN CYANIDE LISTING BACKGROUND DOCUMENT FOR THE INORGANIC CHEMICAL LISTING DETERMINATION August 2000 U.S. ENVIRONMENTAL PROTECTION AGENCY ARIEL RIOS BUILDING 1200 PENNSYLVANIA AVENUE, N.W. WASHINGTON, D.C. 20460 TABLE OF CONTENTS 1. SECTOR OVERVIEW ....................................................1 1.1 Sector Definition, Facility Names and Locations ............................1 1.2 Products, Product Usage and Markets ...................................2 1.3 Production Capacity .................................................4 1.4 Production, Product and Process Trends .................................4 2. DESCRIPTION OF MANUFACTURING PROCESS ...........................5 2.1 Andrussow Process .................................................5 2.2 Variations to the Andrussow Process ....................................6 3. WASTE GENERATION AND MANAGEMENT ...............................8 3.1. Summary of Waste Generation Processes .................................8 3.2.1 Commingled Wastewaters .....................................11 3.2.2 Ammonia Recycle Cartridge and Spent Carbon Filters ................24 3.2.3 Biological Wastewater Treatment Solids ...........................29 3.2.4 Feed Gas Cartridge and Spent Carbon Filters .......................32 3.1.5 Process Air Cartridge Filters ...................................34 3.1.6 Acid Spray Cartridge Filters ....................................35 3.1.7 Spent Catalyst ..............................................36 3.1.8 Ammonium Sulfate and Ammonium Phosphate ......................38 -
Open Dissertation 1-13-2009.Pdf
The Pennsylvania State University The Graduate School Eberly College of Science SYNTHETIC ORGANIC CHEMISTRY AT THE NANOPARTICLE MONOLAYER SOLUTION INTERFACE A Dissertation in Chemistry by Christopher Jay Thode 2009 Christopher Jay Thode Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2009 The dissertation of Christopher Jay Thode was reviewed and approved* by the following: Mary Elizabeth Williams Associate Professor of Chemistry Dissertation Advisor Chair of Committee Thomas E. Mallouk Dupont Professor of Materials Chemistry and Physics Scott T. Philips Assistant Professor of Chemistry Richard Ordway Associate Professor of Biology Ayusman Sen Professor of Chemistry Head, Department of Chemistry *Signatures are on file in the Graduate School iii ABSTRACT As the fields of chemistry, biology, materials science and physics converge upon one another the demands for new materials and technologies continue to run high. Nanotechnology continually emerges as a plausible area of research in which these demands can be met. Specifically, the role of magnetic nanoparticles in new sensors, biomedical, materials and separations technologies has seen extraordinary growth over the past 15 years. With this growth has also emerged new expectations of the control necessary to form these advanced materials and the challenges faced with their syntheses. For magnetic nanoparticles these challenges exist in the realm of particle surface chemistry and functionalization. The field as a whole has given enormous attention to the shape, size and monodispersity of magnetic nanomaterials while leaving their surface functionality comparatively untouched. The following chapters seek to address the development of new general surface chemistries for monolayer protected nanoparticles while envisioning their eventual adaptation to magnetic nanoparticles. -

Formaldehyde (Revised)
uniti slalm Otiicc of Air Quaky EPA-450/4-9 l-012 Fa”irmmenlid Prolccnan planningAnd Standards March 1991 Awncy Rcrurch Trianale Park. NC 2i711 AIR \“f EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF FORMALDEHYDE (REVISED) “, ~.,.~,.,~. .~..~.: ~..~.,, ,..~,‘. .;.. .~_. I < I :...;,.....;&;~~~ ,, ., ~_;_ ..,. .,.~..“_,..,..,. -l-s=h -__- EPA-450/4-91-012 LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF FORMALDEHYDE (REVISED) By Emission Inventory Branch Technical Support Division EPA Project Officer: Dallas Safriet U. S. Environmental Protection Agency Office of Air And Radiation Office of Air Quality Planning and Standards Research Triangle Park, NC 27711 March 1991 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and approved for publication as received from the contractor. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, neither does mention of trade names or commercial products constitute endorsement or recommendation for use. CONTENTS Figures ..................... iv Tables ..................... v 1. Purpose of Document .............. 1 2. Overview of Document Contents ......... 3 3. Background ................... 5 Nature of Pollutant .............. 5 Overview of Production and Uses ........ 8 4. Formaldehyde Emission Sources ......... 13 Formaldehyde Production ............ 13 Urea-Formaldehyde and Melamine-Formaldehyde Resin Production ............... 23 Phenol-Formaldehyde Resin Production -

Chelation Therapy in the Treatment of Metal Intoxication Page Left Intentionally Blank Chelation Therapy in the Treatment of Metal Intoxication
Chelation Therapy in the Treatment of Metal Intoxication Page left intentionally blank Chelation Therapy in the Treatment of Metal Intoxication Jan Aaseth Department of Public Health Hedmark University College, Elverum Department of Internal Medicine Innlandet Hospital, Kongsvinger Hedmark, Norway Guido Crisponi Department of Chemical and Geological Sciences University of Cagliari Cagliari, Italy Ole Andersen Department of Science and Environment Roskilde University Roskilde, Denmark AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an Imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London EC2Y 5AS, UK 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, USA The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Copyright © 2016 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, elec- tronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treat- ment may become necessary. -

Locating and Estimating Sources of Cyanide Compounds
Unitsd States Office of Air (lulity EPA-454/R-93-041 Envkonmsntal Protection Plsnnlng And Standard. AWWV Rawarch Trianals Park. NC 27711 September 1993 PRELIMINARY DATA SEARCH REPORT FOR LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF CYANIDE COMPOUNDS EPA-452/R-93-041 September 1993 PRELIMINARY DATA SEARCH REPORT FOR LOCATING AND ESTIMATING AIR TOXIC EMISSIONS FROM SOURCES OF CYANIDE COMPOUNDS Prepared for: Anne Pope Office of Air Quality Planning and Standards Technical Support Division U. S. Environmental Protection Agency Research Triangle Park, N.C. 27711 Prepared by: Midwest Research Institute 401 Harrison Oaks Boulevard, Suite 350 Cary, North Carolina 27513 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and has been approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. EPA 454/R-93-041 TABLE OF CONTENTS Section Page 1 PURPOSE OF DOCUMENT ................. 1-1 2 OVERVIEW OF DOCUMENT CONTENTS ............ 2-1 3 BACKGROUND ..................... 3-1 3.1 NATURE OF THE POLLUTANT .............. 3-1 3.1.1 Hydrogen Cyanide ............... 3-1 3.1.2 Sodium Cyanide ................ 3-3 3.1.3 Potassium Cyanide ............... 3-3 3.1.4 Other Cyanide Compounds ............ 3-3 3.2 OVERVIEW OF PRODUCTION, USE, AND EMISSIONS OF CYANIDES ...................... 3-6 3.2.1 Production .................. 3-6 3.2.2 Uses ..................... 3-7 3.2.3 Emissions ................... 3-9 4 EMISSIONS FROM PRODUCTION OF MAJOR CYANIDE COMPOUNDS 4-1 4.1 HYDROGEN CYANIDE PRODUCTION ............ 4-1 4.1.1 Process Descriptions ............. 4-3 4.1.2 Emission Control Measures ..........