Using Flow Cytometry and Light-Induced Fluorescence To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U.S. Department of Transportation
234144· REPORT NO. FRA-OR&D-75-54 PB244532 1111111111111111111111111111 FIELD EVALUATION OF LOCOMOTIVE CONSPICUITY LIGHTS D.8. Devoe C.N. Abernethy . :~ . • REPRODUCED BY U.S. DEPARTMENT OF .COMMERCE NATIONAL TECHNICAL INFORMATiON SERVICE SPRINGFIELD, VA 22161 MAY 1975 FINAL REPORT DOCUMENT IS AVAILABLE TO THE PUBLIC THROUGH THE NATIONAL TECHNICAL INFORMATION SERVICE, SPRINGFIELD, VIRGINIA 22161 Prepared for U.S. DEPARTMENT OF TRANSPORTATION FEDERAL RAILROAD ADMINISTRATION Office of Research and Development Washington DC 20590 NOTICE This document is disseminated under the sponsorship of the Department of Transportation in the interest of information exchange. The United States Govern ment assumes no liability for its contents or use thereof. \ \ NOTICE The United States Government does not endorse products or manufacturers. Trade or manufacturers' names appear herein solely because they are con sidered essential to the object of this report. Technical keport Documentation Page 1. Report No. 2. Governmenl Accession No. FRA-OR&D-75-54 t--:~;-,--....,...,.....,......--:-------_..L.-_--------------f'-,~'.:---:--:::---':"'-':"'-'---""~-""--'-'----'---1 4. T,ll_ and Subtitle 5. Report Date FIELD EVALUATION OF LOCOMOTIVE CONSPICUITY May 1975 LIGHTS 6. Performing Organization C"de h~:--:""""'"7-;---'-----------------------j8. Performing Organi zation Report No. 7. Author l s) D.B. Devoe CN AbernethY DOT-TSC-FRA-74-11 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) U.S. Department of Transportation RR402/R 5331 Transportation Systems Center 11. Contract or Grant No. Kendall Square Cambridge MA 02142 12. Sponsoring A.gency Name and Address Final Report U.S. Depar~ment of Transportation March - June 1974 Federal Railroad Administration Office of Research and Development 14. -

DIY Kit 14 - 240V MAINS STROBOSCOPIC LIGHT
DIY Kit 14 - 240V MAINS STROBOSCOPIC LIGHT INTRODUCTION The switch which “closes” to give the pulse of energy to This kit contains the circuit to trigger a xenon flashtube. trigger the xenon flashtube is the neon tube. Let us This flashtube is exactly the same as those seen on aircraft discuss the operation of the neon tube in general before and signal beacons and as those contained in camera flash we look at the circuit in particular. units, fast passport photo kiosks and at discos. Other uses include endoscopes, laser pumps, high speed The neon is connected as a relaxation oscillator as shown photocopiers and typsetting. The frequency of flash can in Figure 1. The neon tube itself can be seen simply to be adjusted from about once every 3 seconds to about 3 contain two electrodes in parallel to each other in a small per second. glass bulb. The air has been replaced by neon gas. When a potential difference (PD) below a critical value is applied (Actually the kit contains TWO flashtubes. The xenon across the electrodes the neon gas will ionize but conduct filled tube is the one the makes all the light. However there almost no current.As the PD approaches the critical value is another flashtube which contains neon gas. It flashes as the neon gas glows with its characteristic orange/pink well but provides a different function as will be explained colour. At about 70V (called the striking voltage) current later.) will flow across the electrodes. The PD must drop to about 60V (the extinction voltage) for current to stop flowing. -

GE Consumer & Industrial
GE Consumer & Industrial LIGHTING GE Consumer & Industrial specialty 2004⁄2005 LAMP CATALOG Specialty Lighting Lamp Products Catalog 2004/2005 GE imagination at work 000 Cover_Ideas_06 2 07/09/04, 11:56 AM 000 Cover_Ideas_06 1 07/09/04, 11:56 AM Introduction SPECIALTY Introduction This catalog lists and provides essential technical data for available General Electric lamps that are used in lighting for specialty markets worldwide including Stage/Studio/TV, Projection/Photo, Sealed Beams, Fluorescent, Incandescent and Discharge Lamps optimized for specific applications. Applications can be severe service (cold, vibration, accessibility), architectural (color, black light), industrial (appliances, germicidal, safety, low voltage, infrared/heat), transportation (aircraft, railroad, marine), and infrastructure (airport, emergency building lighting, traffic signal, sign). Lamp listings are grouped into market/application sections, each containing a “family” of lamps by application or commonalities (such as base, shape, spectral distribution, color temperature), to assist in selection or interchange. Ordering Lamps To order lamps use the GE Order Code, Description and Case Quantity columns. If a lamp is colored BLUE it is stocked in Europe, GREEN is Europe and North America, BLACK is North America only. Otherwise procurement must be through an international distributor or your GE sales representative. North America, European and International sales offices are in the appendix. Other GE Publications All the lamps in this Specialty Catalog come from other GE catalogs/websites. These catalogs and websites contain data for other lamps that may be of interest: In North America: • Lamp Products Catalog (PC 25265) • Miniature/Sealed Beam Catalog (PC 20699) • Stage and Studio SHOWBIZ (PC23766) • www.GELighting.com • or 1-800-GELAMPS In Europe: • GE Consumer and Industrial Lighting Lamp Catalogue-Spectrum • SHOWBIZ® (ENTCAT 02/2003) Lamp Index There is a sorted (numeric/alphabetic) index by description with ANSI/LIF code, if available, which provides page number. -

User Manual 2.3 MB
10M 25M 50M 75M 100M 10Y 25Y 50Y 75Y 100Y impact TM For EX-100A accessories and to see all of our lighting equipment, please visit our Web site. impactTM EX-100A Monolight www.impactstudiolighting.com INSTRUCTIONS Page 20 Page 1 (back cover) (front cover) 10M 25M 50M 75M 100M 10Y 25Y 50Y 75Y 100Y Thank you for your purchase of the Impact EX-100A Monolight. The One-Year Limited Warranty EX-100A Monolight is economical and lightweight, yet durable enough to give you many years of trouble-free service and enjoyment. Please read these operating instructions and safety precautions carefully before operating this equipment. Features • Three-stop range – full power to 1/8 power, steplessly • Built-in optical slave • Modeling lamp can be set to proportional or full power • Accepts Elinchrom-style reectors and head accessories (8-inch grid reector included) • Tactile, “grippy” feel that resists slipping, scratches, and shock damage • Commonly available 1/8˝ mini-plug sync input • Low 4.3V trigger voltage – safe for any camera’s circuitry Power Requirements This light comes in two models; one is designed for use with 110/120V AC power in the US and the other for 220V AC power in Europe. Neither model can be used outside of its native power region. Both are supplied with a three-prong, grounded plug. Do not attempt to defeat this safety feature. If necessary, use only grounded extension cords rated for six amps or greater. Warning There are no user-serviceable parts inside the unit. Only qualied service engineers should access the inside of the case (Danger – high-voltage parts inside). -

Ultraviolet Radiation
Environmental Health Criteria 160 Ultraviolet Radiation An Authoritative Scientific Review of Environmental and Health Effects of UV, with Reference to Global Ozone Layer Depletion V\JflVV ptiflcti1p cii ii, L?flUctd EnrrcmH Prormwe. Me World Haah6 Orgniri1ion and Fhc nIrrHbccrlT Ornrn)is5ion on Nfl-oflizirig Raditiori Prioiioii THE Ef4VIRONMEF4FAL HEALTH CI4ITERIA SERIES Acetonitrile (No. 154, 1993) 2,4-Dichloroplierioxyaceric acid (2 4 D) (No 29 Acrolein (No 127, 1991) 1984) Acrylamide (No 49, 1985) 2,4.Dichlorophenoxyucetic acd - erivirorrmerrtul Acr5lonilrile (No. 28, 1983) aspects (No. 54, 1989) Aged population, principles for evaluating the 1 ,3-Dichloroproperte, 1,2-dichloropropane and effects of chemicals (No 144, 1992) mixtures (No. 146, 1993( Aldicarb (No 121, 1991) DDT and its derivatives (No 9 1979) Aidrin and dieldrin (No 91 1989) DDT and its derivatives - environmental aspects Allethrins (No 87, 1989) (No. 83, 1989) Alpha-cypermethrirr (No 142, 1992) Deltamethrin (No 97, 1990) Ammonia (No 54, 1985) Diamirrotoluenes (No 74, 1987( Arsenic (No 18. 1981) Dichiorsos (No. 79, 1988) Asbestos and other natural mineral fibres Diethylhexyl phthalate (No. 131, 191112) (No. 53, 198€) Dirnethoate (No 90, 1989) Barium (No. 137 1990) Dimethylformnmde (No 114, 1991) Benomy( (No 143, 1993) Dimethyf sulfate (No. 48. 1985) Benzene (No 150, 1993) Diseases of suspected chemical etiology and Beryllium (No 106, 1990( their prevention principles of studies on Biommkers and risk assessment concepts (No. 72 1967) and principles (No. 155, 1993) Dilhiocarbsmats pesticides, ethylerrvthiourea, and Biotoxins, aquatic (marine and freshmaterl propylerrethiourea a general introdUCtiori (No 37, 1984) NO. 78. 1958) Butanols . four isomers (No. 65 1987) Electromagnetic Fields (No 1 '37 19921 Cadmiurrr (No 134 1992) Endosulfan (No 40. -

Photogenic 2Catalog.Qxd
® A Brand of Products and Accessories Catalog SolairTM Constant Color PowerLights® with PocketWizard™ Radio Triggering Photogenic® and PocketWizardTM combine technologies to bring you wireless triggering, wireless power control and constant Kelvin temperatures built into SOLAIR® series PowerLights® PLR1000DRC (958438) Radio SolairTM (1000 watt-seconds) Wireless Triggering All “DR” and SolairTM Constant-Color models are now available with 32 channel radio technology that wirelessly trigger your strobes up to a distance of 1600 feet. You can use multiple lights in the studio and on location and never worry about your lights being triggered by another photographer’s flash. No more unreliable sync cords. No more broken or lost antennas. The antenna is built into the light. Improve your digi- tal images immediately by eliminating sync cord line noise. Position your lights anywhere, not just where the sync cord reaches. It’s easy. Just set the radio PowerLight® to one of 32 channels on the Pocket Wizard transmitter, and you are ready for wireless triggering. The big advantage is the built-in radio receiver never needs batteries. The Plus receivers from PocketWizard™ are fully compatible with all other PocketWizard transmit- ters. This includes the transmitters that are built into Kodak®, Nikon®, Mamiya® cameras and Sekonic® meters, as well as Standard Plus and MultiMax units. For complete wireless control, use Photogenic® radio ready PowerLights® and SolairTM Constant- ® TM Color lights with the Photogenic Infrared Remote Solair Radio Built-in -

FTS 430/830-4 Approach Lighting System Reference Manual
FTS 430/830-4 Approach Lighting System Reference Manual Part Number F791430830 SERIAL NUMBER Flash Technology, 332 Nichol Mill Lane, Franklin, TN 37067 www.flashtechnology.com (615) 261-2000 Front Matter Abstract This manual contains information and instructions for installing, operating and maintaining the FTS 430 and FTS 830 Approach Lighting Systems. Copyright Copyright© 2018, Flash Technology®, Franklin, TN, 37067, U.S.A. All rights reserved. Reproduction or use of any portion of this manual is prohibited without express written permission from Flash Technology and/or its licenser. Trademark Acknowledgements Flash Technology is a registered trademark of SPX Corporation. All trademarks and product names mentioned are properties of their respective companies, and are recognized and acknowledged as such by Flash Technology. Applicable Specifications This equipment meets or exceeds requirements for an FAA Type L-849 Style A and E, and Type L-859, Styles B and F. Disclaimer While every effort has been made to ensure that the information in this manual is complete, accurate and up-to-date, Flash Technology assumes no liability for damages resulting from any errors or omissions in this manual, or from the use of the information contained herein. Flash Technology reserves the right to revise this manual without obligation to notify any person or organization of the revision. In no event will Flash Technology be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of or the inability to use this manual. Warranty Flash Technology warrants all components, under normal operating conditions, for two years. Parts Replacement The use of parts or components, in this equipment, not manufactured or supplied by Flash Technology voids the warranty and invalidates the third party testing laboratory certification which ensures compliance with FAA Advisory Circulars 150/5345-51. -

Abls Study Guide
______________________________________ Excerpts from the ABLS STUDY GUIDE (The complete Study Guide is used as part of the ABLS Certification process. These excerpts are for use in certain seminars and courses on medical lasers that the ABLS may be associated with, but is not a substitute for the actual ABLS Certification process. Information about the Board’s Certification is available at the ABLS website: www.americanboardoflasersurgery.org) _____________________________________ © The American Board of Laser Surgery Inc., 2016. All Rights Reserved. No part of these Study Guide Excerpts may be reproduced in any form without the express written consent of the ABLS. © The American Board of Laser Surgery Inc., 2016. All Rights Reserved. 1 Introduction ---- CHAPTER 1 Fundamentals of Laser Physics, Optics and Operating Characteristics for the Clinician ---- CHAPTER 2 Surgical Delivery Systems ---- CHAPTER 3 Laser Biophysics, Tissue Interaction, Power Density and Ablative Resurfacing of Human Skin: Essential Foundations for Laser Dermatology and Cosmetic Procedures ---- CHAPTER 4 Commentary on Ethics in Cosmetic Laser Surgery ---- CHAPTER 5 Safe Use of Lasers in Surgery ---- CHAPTER 6 Considerations in the Selection of Equipment © The American Board of Laser Surgery Inc., 2016. All Rights Reserved. 2 Contents and Topics in the Study Chapter 4 5 Materials : Commentary on Ethics in Cosmetic Laser Surgery (key considerations in providing Coptihamptuemr 5patient care). The study material consists of the proprietary ABLS Study Guide on fundamental laser science, : The Safe Use of Lasers in Surgery Fordelivery cosmetic systems, practitioners biophysics/ only tissue interaction, (oriented to the needs of the actual practitioner ethics, laser safety, and equipment selection. aCsh oappopterse d6 t:o support personnel) it also includes several chaptersLasers from and two Lights: excellent Procedures books on in a Considerations in the Selection broad range of cosmeticnd laser and light Cosmetic Dermatology, 2 Ed. -
Gp3/Mclr/Vpp Gp2/Tocks
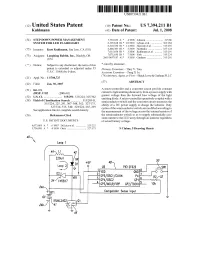
USOO7394211B1 (12) United States Patent (10) Patent No.: US 7,394.211 B1 Kuhlmann (45) Date of Patent: Jul. 1, 2008 (54) STEP DOWN POWER MANAGEMENT 5,739,639 A 4, 1998 Johnson ....................... 315,86 SYSTEM FOR LED FLASHLIGHT 6,305,818 B1 * 10/2001 Lebens et al. ..... ... 362, 184 6,320,330 B1 * 1 1/2001 Haavisto et al. ... ... 315,291 (75) Inventor: Kurt Kuhlmann, San Jose, CA (US) 6,888,383 B1* 5/2005 Fairbanks ............ ... 327,124 7,015,654 B1* 3/2006 Kuhlmann et al. ... 315,291 7,071,630 B1* 7/2006 York ...... 315,224 (73) Assignee: Fashing Rabbit, Inc., Blachly, OR 2005/0057187 A1 3/2005 Catalano ..................... 315,291 (*) Notice: Subject to any disclaimer, the term of this * cited by examiner patent is extended or adjusted under 35 Primary Examiner Thuy V. Tran U.S.C. 154(b) by 0 days. Assistant Examiner Tung X Le (74) Attorney, Agent, or Firm Black Lowe & Graham PLLC (21) Appl. No.: 11/700,727 57 ABSTRACT (22) Filed: Jan. 30, 2007 (57) (51) Int. Cl A micro-controller and a converter circuit provide constant irosh 3702 (2006.01) current to light emitting diode array from a power Supply with greater Voltage than the forward bias Voltage of the light (52) U.S. Cl. ......... r 315/291; 315/224; . 5/362 emitting diode. A micro-controller operatively coupled with a (58) Field of Classification Search ............. 315/209 R, semiconductor Switch and the converter circuit measures the 315/224,327/514-515,540; 225, 291, 307 308, 323/222, 362; 327/175,265, 299 ability of a DC power supply to charge the inductor. -

Analysis and Design of a New Control System for Aerodrome Azimuthal Guidance – the SAGA
Analysis and design of a new control system for aerodrome azimuthal guidance – The SAGA Analysis and design of a new control system for aerodrome azimuthal guidance – The SAGA Joakim Arnsby, Mårten Kjellsson Department of Automatic Control Department of Automatic Control Lund University Box 118 SE-221 00 LUND Sweden ISSN 0280–5316 ISRN LUTFD2/TFRT--5920--SE c 2013 by Joakim Arnsby, Mårten Kjellsson. All rights reserved. Printed in Sweden by Media-Tryck. Lund 2013 Abstract This master thesis work consists of the development of new hardware and software for the control box of an azimuthal guidance system used by air- crafts during the approach to the runway. The thesis work was done at Safegate International AB as a project running along the usual development projects. When designing electronics to be used on an airfield there are a lot of regulations and standards that need to be taken into consideration. To en- sure the project turned out to match the demands, some project manage- ment documents where drafted, both documents that specified requirements and documents that specified different tests to ensure the prototype did what it was supposed to. As the project finalized, two prototypes of control boxes were installed and working on one azimuthal guidance system. These prototypes included full documentation and changes to existing manuals to be able to make the new control boxes part of the Safegate experience. i Acknowledgements We would like to thank our supervisors, Andreas Wirandi and Mattias Hall- berg at Safegate and Anders Robertsson at LTH, for all their help with the project management and expertise in their different fields. -

LED Flicker Measurement: Challenges, Considerations, and Updates from IEEE P2020 Working Group
https://doi.org/10.2352/ISSN.2470-1173.2020.16.AVM-001 © 2020, Society for Imaging Science and Technology LED flicker measurement: Challenges, considerations, and updates from IEEE P2020 working group Brian Michael Deegan; Valeo Vision Systems; Tuam, Ireland Abstract assist camera), LED flicker may be considered as an annoyance or The introduction of pulse width modulated LED lighting in at worst a distraction for the driver. However for CMS (i.e. mirror automotive applications has created the phenomenon of LED replacement) cameras, flickering headlamps may be mistaken for flicker. In essence, LED flicker is an imaging artifact, whereby a turn signals/indicators or, as has been reported, may cause the driver light source will appear to flicker when image by a camera system, to misidentify a following vehicle as an emergency vehicle. For even though the light will appear constant to a human observer. The machine vision based ADAS or autonomous driving applications, implications of LED flicker vary, depending on the imaging the consequences may be even more severe. LED flicker may cause application. In some cases, it can simply degrade image quality by misidentification of traffic signals, speed signs or safety messages. causing annoying flicker to a human observer. However, LED In this paper, a brief overview of the root cause of LED flicker flicker has the potential to significantly impact the performance of is provided. The implications of LED on various automotive use critical autonomous driving functions. In this paper, the root cause cases is explored. Guidelines on measurement and assessment of of LED flicker is reviewed, and its impact on automotive use cases LED flicker are also provided. -

F a a Advisory Circular 150/5345-43H, Specification For
U.S. Department Advisory of Transportation Federal Aviation Circular Administration Subject: Specification for Obstruction Lighting Date: 9/28/2016 AC No: 150/5345-43H Equipment Initiated By: AAS-100 Change: 1 Purpose. This advisory circular (AC) contains the Federal Aviation Administration (FAA) specification for obstruction lighting equipment. 2 Effective Date. Effective 12 months after the date of this circular, only that equipment qualified per this specification will be listed in AC 150/5345-53, Airport Lighting Equipment Certification Program. No re-testing will be required for existing equipment where test standards are unchanged from the previous version of this AC. 3 Cancellation. This AC cancels AC 150/5345-43G, Specification for Obstruction Lighting Equipment, dated September 26, 2006. 4 Application. The Federal Aviation Administration (FAA) recommends the guidance and specifications in this Advisory Circular for Obstruction Lighting Equipment. In general, use of this AC is not mandatory. However, the use of the specifications in this AC is mandatory for lighting or projects funded under the Airport Improvement Program (AIP) or with revenue from the Passenger Facility Charges (PFC) program. All lighting designs contained in this AC are acceptable to the Administrator to meet the lighting requirements under Title 14 § 139.311, Marking, Signs and Lighting. 5 Principal Changes. The AC incorporates the following principal changes: 9/28/2016 AC 150/5345-43H 1. Long paragraphs in various sections are split up into separate list items to facilitate better comprehension. 2. Paragraph 1.2 – L810(F) light is added. 3. Chapter 2 updates all Internet links for referenced documents. 4. Paragraph 3.3.4 is added for obstruction light mounting provisions.