The Reproductive Cycle and Development of Crepipatella Fecunda (Gastropoda: Calyptraeidae) from Southern Chile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crepidula Fornicata
NOBANIS - Marine invasive species in Nordic waters - Fact Sheet Crepidula fornicata Author of this species fact sheet: Kathe R. Jensen, Zoological Museum, Natural History Museum of Denmark, Universiteteparken 15, 2100 København Ø, Denmark. Phone: +45 353-21083, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Jensen, Kathe R. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Crepidula fornicata – From: Identification key to marine invasive species in Nordic waters – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Species name Crepidula fornicata, Linnaeus, 1758 – Slipper limpet Synonyms Patella fornicata Linnaeus, 1758; Crepidula densata Conrad, 1871; Crepidula virginica Conrad, 1871; Crepidula maculata Rigacci, 1866; Crepidula mexicana Rigacci, 1866; Crepidula violacea Rigacci, 1866; Crepidula roseae Petuch, 1991; Crypta nautarum Mörch, 1877; Crepidula nautiloides auct. NON Lesson, 1834 (ISSG Database; Minchin, 2008). Common names Tøffelsnegl (DK, NO), Slipper limpet, American slipper limpet, Common slipper snail (UK, USA), Østerspest (NO), Ostronpest (SE), Amerikanische Pantoffelschnecke (DE), La crépidule (FR), Muiltje (NL), Seba (E). Identification Crepidula fornicata usually sit in stacks on a hard substrate, e.g., a shell of other molluscs, boulders or rocky outcroppings. Up to 13 animals have been reported in one stack, but usually 4-6 animals are seen in a stack. Individual shells are up to about 60 mm in length, cap-shaped, distinctly longer than wide. The “spire” is almost invisible, slightly skewed to the right posterior end of the shell. Shell height is highly variable. Inside the body whorl is a characteristic calcareous shell-plate (septum) behind which the visceral mass is protected. -

The American Slipper Limpet Crepidula Fornicata (L.) in the Northern Wadden Sea 70 Years After Its Introduction
Helgol Mar Res (2003) 57:27–33 DOI 10.1007/s10152-002-0119-x ORIGINAL ARTICLE D. W. Thieltges · M. Strasser · K. Reise The American slipper limpet Crepidula fornicata (L.) in the northern Wadden Sea 70 years after its introduction Received: 14 December 2001 / Accepted: 15 August 2001 / Published online: 25 September 2002 © Springer-Verlag and AWI 2002 Abstract In 1934 the American slipper limpet 1997). In the centre of its European distributional range Crepidula fornicata (L.) was first recorded in the north- a population explosion has been observed on the Atlantic ern Wadden Sea in the Sylt-Rømø basin, presumably im- coast of France, southern England and the southern ported with Dutch oysters in the preceding years. The Netherlands. This is well documented (reviewed by present account is the first investigation of the Crepidula Blanchard 1997) and sparked a variety of studies on the population since its early spread on the former oyster ecological and economic impacts of Crepidula. The eco- beds was studied in 1948. A field survey in 2000 re- logical impacts of Crepidula are manifold, and include vealed the greatest abundance of Crepidula in the inter- the following: tidal/subtidal transition zone on mussel (Mytilus edulis) (1) Accumulation of pseudofaeces and of fine sediment beds. Here, average abundance and biomass was 141 m–2 through the filtration activity of Crepidula and indi- and 30 g organic dry weight per square metre, respec- viduals protruding in stacks into the water column. tively. On tidal flats with regular and extended periods of This was reported to cause changes in sediments and emersion as well as in the subtidal with swift currents in near-bottom currents (Ehrhold et al. -

Metagenetic Analysis of 2017 Plankton Samples from Prince William Sound, Alaska
Metagenetic Analysis of 2017 Plankton Samples from Prince William Sound, Alaska. Report to Prince William Sound Regional Citizens’ Advisory Council (PWSRCAC) From Molecular Ecology Laboratory Moss Landing Marine Laboratory Dr. Jonathan Geller Melinda Wheelock Martin Guo Any opinions expressed in this PWSRCAC-commissioned report are not necessarily those of PWSRCAC. December 1, 2018 Revised August 15, 2019 Abstract This report describes the methods and findings of the metagenetic analysis of plankton samples from the waters of Prince William Sound (PWS), Alaska. The study was done to identify zooplankton, in particular the larvae of invasive benthic species. Plankton samples, collected by the Prince William Sound Science Center (PWSSC), were analyzed by the Molecular Ecology Laboratory at the Moss Landing Marine Laboratories. The samples were taken from five stations in May of 2017 in Port Valdez and elsewhere in PWS. DNA was extracted from bulk plankton and a portion of the mitochondrial Cytochrome c oxidase subunit 1 gene, the most commonly used DNA barcode for animals, was amplified by polymerase chain reaction (PCR). Products of PCR were sequenced using Illumina reagents and MiSeq instrument. 211 operational taxonomic units (an approximation of biological species) were found and 52 were identified to species. Most species were crustaceans and molluscs, and none were non-native. We also compared PWSRCAC samples taken in 2016 to the current set of samples. Fewer species were identified in 2017 than in 2016, but sampling methods varied across years. Standardization of methods and a longer time series are necessary to investigate temporal trends. Page 1 of 17 952.431.190815.MLMetagenetic Introduction Monitoring marine habitat for species of concern, including invasive species, can be costly and time-consuming, which limits the information available to resource managers, scientists, and the public. -

Research Article Early Development of Monoplex Pilearis
1 Research Article 2 Early Development of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: 3 Cymatiidae) - Biology and Morphology 4 5 Ashlin H. Turner*, Quentin Kaas, David J. Craik, and Christina I. Schroeder* 6 7 Institute for Molecular Bioscience, The University of Queensland, Brisbane, 4072, Qld, Australia 8 9 *Corresponding authors: 10 Email: [email protected], phone: +61-7-3346-2023 11 Email: [email protected], phone: +61-7-3346-2021 1 12 Abstract 13 Members of family Cymatiidae have an unusually long planktonic larval life stage (veligers) which 14 allows them to be carried within ocean currents and become distributed worldwide. However, little 15 is known about these planktonic veligers and identification of the larval state of many Cymatiidae 16 is challenging at best. Here we describe the first high-quality scanning electron microscopy images 17 of the developing veliger larvae of Monoplex pilearis and Monoplex parthenopeus (Gastropoda: 18 Cymatiidae). The developing shell of Monoplex veligers was captured by SEM, showing plates 19 secreted to form the completed shell. The incubation time of the two species was recorded and 20 found to be different; M. parthenopeus took 24 days to develop fully and hatch out of the egg 21 capsules, whereas M. pilearis took over a month to leave the egg capsule. Using scanning electron 22 microscopy and geometric morphometrics, the morphology of veliger larvae was compared. No 23 significant differences were found between the shapes of the developing shell between the two 24 species; however, it was found that M. pilearis was significantly larger than M. -

Download Preprint
1 Mobilising molluscan models and genomes in biology 2 Angus Davison1 and Maurine Neiman2 3 1. School of Life Sciences, University Park, University of Nottingham, NG7 2RD, UK 4 2. Department of Biology, University of Iowa, Iowa City, IA, USA and Department of Gender, 5 Women's, and Sexuality Studies, University of Iowa, Iowa, City, IA, USA 6 Abstract 7 Molluscs are amongst the most ancient, diverse, and important of all animal taxa. Even so, 8 no individual mollusc species has emerged as a broadly applied model system in biology. 9 We here make the case that both perceptual and methodological barriers have played a role 10 in the relative neglect of molluscs as research organisms. We then summarize the current 11 application and potential of molluscs and their genomes to address important questions in 12 animal biology, and the state of the field when it comes to the availability of resources such 13 as genome assemblies, cell lines, and other key elements necessary to mobilising the 14 development of molluscan model systems. We conclude by contending that a cohesive 15 research community that works together to elevate multiple molluscan systems to ‘model’ 16 status will create new opportunities in addressing basic and applied biological problems, 17 including general features of animal evolution. 18 Introduction 19 Molluscs are globally important as sources of food, calcium and pearls, and as vectors of 20 human disease. From an evolutionary perspective, molluscs are notable for their remarkable 21 diversity: originating over 500 million years ago, there are over 70,000 extant mollusc 22 species [1], with molluscs present in virtually every ecosystem. -

Fecundity of the Invasive Marine Gastropod Crepidula Fornicata Near the Current Northern Extreme of Its Range
Invertebrate Biology 136(4): 394–402. © 2017, The American Microscopical Society, Inc. DOI: 10.1111/ivb.12194 Fecundity of the invasive marine gastropod Crepidula fornicata near the current northern extreme of its range Jan A. Pechenik,1,a Casey M. Diederich,1 Howard I. Browman,2 and Anders Jelmert3 1 Department of Biology, Tufts University, Medford, Massachusetts 02155, USA 2 Institute of Marine Research, Austevoll Research Station, Storebø, Norway 3 Institute of Marine Research, 5817 Bergen, Norway Abstract. The calyptraeid gastropod Crepidula fornicata is native to the eastern coast of the United States but has now become an extremely successful invader along much of the Euro- pean coastline. As the northern limit of its spread is thought to be determined by an inabil- ity of adults to tolerate prolonged exposure to low winter temperatures, this study sought to compare the fecundity of females collected from two sites along the Norwegian coastline with that of females collected from Rhode Island, USA. Few other studies have compared the fecundities of marine invertebrates from invasive populations with those found in native populations. For both populations studied, fecundities increased with increasing shell length. However, contrary to expectations, size-related fecundities were significantly higher for Norwegian females than for Rhode Island females, with Norwegian females producing larger egg capsules and a greater number of embryos per capsule, but not a greater number of egg capsules per brood. Current evidence suggests that at -

Tesis De Pablo David Vega García
Programa de Estudios de Posgrado CAMBIOS HISTÓRICOS EN LAS POBLACIONES DE ABULÓN AZUL Y AMARILLO EN LA PENÍNSULA DE BAJA CALIFORNIA TESIS Que para obtener el grado de Doctor en Ciencias Uso, Manejo y Preservación de los Recursos Naturales Orientación Biología Marina P r e s e n t a PABLO DAVID VEGA GARCÍA La Paz, Baja California Sur, febrero de 2016 COMITÉ TUTORIAL Dr. Salvador Emilio Lluch Cota Director de Tesis Centro de Investigaciones Biológicas del Noroeste. La Paz, BCS. México. Dra. Fiorenza Micheli Dr. Héctor Reyes Bonilla Co-Tutor Co-Tutor Hopkins Marine Station, Stanford Universidad Autónoma de Baja University. California Sur. Pacific Grove, CA. EEUU. La Paz, BCS. México. Dr. Eduardo Francisco Balart Páez Dr. Pablo Del Monte Luna Co-Tutor Co-Tutor Centro de Investigaciones Biológicas Centro de Interdisciplinario de del Noroeste. Ciencias Marinas. La Paz, BCS. México La Paz, BCS. México. COMITÉ REVISOR DE TESIS Dr. Salvador Emilio Lluch Cota Dra. Fiorenza Micheli Dr. Eduardo Francisco Balart Páez Dr. Héctor Reyes Bonilla Dr. Pablo Del Monte Luna JURADO DE EXAMEN Dr. Salvador Emilio Lluch Cota Dra. Fiorenza Micheli Dr. Eduardo Francisco Balart Páez Dr. Héctor Reyes Bonilla Dr. Pablo Del Monte Luna SUPLENTES Dr. Fausto Valenzuela Quiñonez Dr. Raúl Octavio Martínez Rincón RESUMEN El abulón es un importante recurso pesquero en México que en las últimas décadas ha presentado una importante disminución de sus poblaciones, a pesar de las estrictas regulaciones a las que está sometida su explotación. Si bien la tendencia general de las capturas de abulón indica una disminución de las dos principales especies que la conforman (Haliotis fulgens y H corrugata), a partir de su máximo histórico en 1950, esta tendencia no ha sido uniforme ni entre especies ni entre las regiones de donde se extrae. -

Crepidula Formicatacrepidula Formicata
Crepidula formicataCrepidula formicata Easily seen Crepidula fornicata along the shoreline. Class: Gastropoda Order: Littorinimorpha Family: Calyptraeidae Genus: Crepidula Distribution It is native to the eastern This species has been introduced to several other countries, coast of North America. especially in Europe. It is considered to be an invasive, nuisance It occurs from the Gulf of species. The first known occurrence of Crepidula fornicata in St. Lawrence in the north Europe was in 1872. It was introduced accidently with imported all the way south to the American oysters. They have been repeatedly introduced ever Gulf of Mexico. since in ballast water and on ships’ hulls. Habitat They are most abundant in muddy or mixed muddy areas, This species occupies a reaching their highest densities in wave protected locations. variety of seabed surfaces. These include bays, estuaries and sheltered sides of wave- They also occur in a wide exposed islands. In Nova Scotia they are common in intertidal range of environmental and shallow subtidal regions. They attach themselves to almost conditions. any hard surface to be found (including each other and the shells of other living or dead hard-shelled invertebrates. Food They are active suspension feeders, generating a water current They live off of plankton through the mantle cavity by ciliary (hair-like) action and which they filter out of the trapping food particles on a mucous sheet lying across the front seawater. surface of the gill filament. Reproduction Being hermaphrodites they contain both male and female The Slipper limpet is a. reproductive organs. Sperm producing ability advances quicker protandric hermaphrodite. -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

A Comparative Study of Some Population Characteristics Of
Brigham Young University BYU ScholarsArchive Theses and Dissertations 1975-04-01 A comparative study of some population characteristics of Calyptraea fastigiata Gould, Crepidula lingulata Gould, and Crepidula nummaria Gould (Gastropoda, Prosobranchia) Roger Harold Goodwill Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd BYU ScholarsArchive Citation Goodwill, Roger Harold, "A comparative study of some population characteristics of Calyptraea fastigiata Gould, Crepidula lingulata Gould, and Crepidula nummaria Gould (Gastropoda, Prosobranchia)" (1975). Theses and Dissertations. 7935. https://scholarsarchive.byu.edu/etd/7935 This Thesis is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. A COMPARATIVE STUDY OF SOME POPULATION CHARACTERISTICS OF CALYPTRAEA FASTIGIATA GOULD, CREPIDULA LINGULATA GOULD, Pi.ND CREPIDULA NUMM.�IA GOULD (GASTROPODA, PROSOBRANCHIA) A Thesis Presented to the Department of Zoology Brigham Young University In Partial Fulfillment of the Requirements for the Degree Master of Science v by Roger Harold Goodwill April 1975 This thesis, by Roger Harold Goodwill, is accepted in its present form by the Department of Zoology of Brigham Young University as satisfying the thesis requirement for the degree of Master of Science. Typed by Katherine Shepherd ii ACKNOWLEDGMENTS I would like to express my appreciation to Dr. Lee Braithwaite for his help and friendship throughout my grad uate studies at BYU. I also offer my thanks to Dr. James Barnes and Dr. Melvin Carter who gave of their time freely and offered many helpful suggestions. -

1 Metagenetic Analysis of 2018 and 2019 Plankton Samples from Prince
Metagenetic Analysis of 2018 and 2019 Plankton Samples from Prince William Sound, Alaska. Report to Prince William Sound Regional Citizens’ Advisory Council (PWSRCAC) From Molecular Ecology Laboratory Moss Landing Marine Laboratory Dr. Jonathan Geller Melinda Wheelock Martin Guo Any opinions expressed in this PWSRCAC-commissioned report are not necessarily those of PWSRCAC. April 13, 2020 ABSTRACT This report describes the methods and findings of the metagenetic analysis of plankton samples from the waters of Prince William Sound (PWS), Alaska, taken in May of 2018 and 2019. The study was done to identify zooplankton, in particular the larvae of benthic non-indigenous species (NIS). Plankton samples, collected by the Prince William Sound Science Center (PWSSC), were analyzed by the Molecular Ecology Laboratory at the Moss Landing Marine Laboratories. The samples were taken from five stations in Port Valdez and nearby in PWS. DNA was extracted from bulk plankton and a portion of the mitochondrial Cytochrome c oxidase subunit 1 gene (the most commonly used DNA barcode for animals) was amplified by polymerase chain reaction (PCR). Products of PCR were sequenced using Illumina reagents and MiSeq instrument. In 2018, 257 operational taxonomic units (OTU; an approximation of biological species) were found and 60 were identified to species. In 2019, 523 OTU were found and 126 were identified to species. Most OTU had no reference sequence and therefore could not be identified. Most identified species were crustaceans and mollusks, and none were non-native. Certain species typical of fouling communities, such as Porifera (sponges) and Bryozoa (moss animals) were scarce. Larvae of many species in these phyla are poorly dispersing, such that they will be found in abundance only in close proximity to adult populations. -
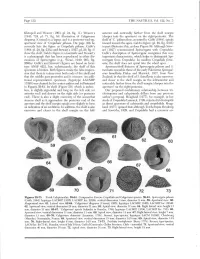
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave.