Left Superior Vena Cava with Left Azygos Vein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF File
Folia Morphol. Vol. 78, No. 2, pp. 283–289 DOI: 10.5603/FM.a2018.0077 O R I G I N A L A R T I C L E Copyright © 2019 Via Medica ISSN 0015–5659 journals.viamedica.pl Observations of foetal heart veins draining directly into the left and right atria J.H. Kim1, O.H. Chai1, C.H. Song1, Z.W. Jin2, G. Murakami3, H. Abe4 1Department of Anatomy and Institute of Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea 2Department of Anatomy, Wuxi Medical School, Jiangnan University, Wuxi, China 3Division of Internal Medicine, Jikou-kai Clinic of Home Visits, Sapporo, Japan 4Department of Anatomy, Akita University School of Medicine, Akita, Japan [Received: 19 June 2018; Accepted: 8 August 2018] Evaluation of semiserial sections of 14 normal hearts from human foetuses of gestational age 25–33 weeks showed that all of these hearts contained thin veins draining directly into the atria (maximum, 10 veins per heart). Of the 75 veins in these 14 hearts, 55 emptied into the right atrium and 20 into the left atrium. These veins were not accompanied by nerves, in contrast to tributaries of the great cardiac vein, and were negative for both smooth muscle actin (SMA) and CD34. However, the epithelium and venous wall of the anterior cardiac vein, the thickest of the direct draining veins, were strongly positive for SMA and CD34, respectively. In general, developing fibres in the vascular wall were positive for CD34, while the endothelium of the arteries and veins was strongly positive for the present DAKO antibody of SMA. -

Azygos Vein System Abnormality: Case Report
Gülhane Týp Dergisi 2006; 48: 180-182 OLGU SUNUMU © Gülhane Askeri Týp Akademisi 2006 Azygos vein system abnormality: case report Necdet Kocabýyýk (*), Tunç Kutoðlu (**), Soner Albay (*), Bülent Yalçýn (*), Hasan Ozan (*) Summary Introduction Variations seen in the thoracic vein system are Abnormalities related to the azygos system are not rare (1). In a series related to the development of these veins. of 200 cases, Bergman et al. have reported the incidence of this anomaly During the dissection from the posterior medi- astinum of the 60-year-old male cadaver, it 26% (2). These abnormalities are generally explained by the embryolog- was observed that there was no complete ical development. Venous branching of the azygos vein varies (3). There accessory hemiazygos vein, and both posterior are two origins of the azygos and hemiazygos veins. By union of these intercostal veins and hemiazygos vein (above origins and regression of some parts, azygos system comes into its final T10 level) drained bilaterally to the azygos vein. Considering these types of variations is status (4). Different types of structures may occur when these veins important during imaging this region and surgi- develop. Abnormalities about azygos system and especially the variations cal operations. of the hemiazygos veins are not clearly described in the literature. In this Key words: Azygos vein, hemiazygos vein, superior vena cava, venous anomaly presentation absence of the accessory hemiazygos vein and possible causes of these types of variations are discussed in view of the embry- Özet ological development. Azigos ven sistem anomalisi: olgu sunumu Toraks ven sisteminde görülen varyasyonlar, embriyolojik olarak bu venlerin geliþimiyle ilgi- Case Report lidir. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Non-Pathological Opacification of the Cavernous Sinus on Brain CT
healthcare Article Non-Pathological Opacification of the Cavernous Sinus on Brain CT Angiography: Comparison with Flow-Related Signal Intensity on Time-of-Flight MR Angiography Sun Ah Heo 1, Eun Soo Kim 1,* , Yul Lee 1, Sang Min Lee 1, Kwanseop Lee 1 , Dae Young Yoon 2, Young-Su Ju 3 and Mi Jung Kwon 4 1 Department of Radiology, Hallym University Sacred Heart Hospital, College of Medicine, Hallym University, Seoul 14068, Korea; [email protected] (S.A.H.); [email protected] (Y.L.); [email protected] (S.M.L.); [email protected] (K.L.) 2 Department of Radiology, Kangdong Sacred Heart Hospital, College of Medicine, Hallym University, Seoul 14068, Korea; [email protected] 3 National Medical Center, Seoul 04564, Korea; [email protected] 4 Department of Pathology, Hallym University Sacred Heart Hospital, College of Medicine, Hallym University, Seoul 14068, Korea; [email protected] * Correspondence: [email protected] Abstract: Purpose: To investigate the non-pathological opacification of the cavernous sinus (CS) on brain computed tomography angiography (CTA) and compare it with flow-related signal intensity (FRSI) on time-of-flight magnetic resonance angiography (TOF-MRA). Methods: Opacification of the CS was observed in 355 participants who underwent CTA and an additional 77 participants who underwent examination with three diagnostic modalities: CTA, TOF-MRA, and digital subtraction angiography (DSA). Opacification of the CS, superior petrosal sinus (SPS), inferior petrosal sinus Citation: Heo, S.A.; Kim, E.S.; Lee, Y.; Lee, S.M.; Lee, K.; Yoon, D.Y.; Ju, Y.-S.; (IPS), and pterygoid plexus (PP) were also analyzed using a five-point scale. -
Endothelial Cell Dynamics in Vascular Development: Insights from Live-Imaging in Zebrafish
fphys-11-00842 July 20, 2020 Time: 12:10 # 1 REVIEW published: 22 July 2020 doi: 10.3389/fphys.2020.00842 Endothelial Cell Dynamics in Vascular Development: Insights From Live-Imaging in Zebrafish Kazuhide S. Okuda1,2 and Benjamin M. Hogan1,2,3* 1 Organogenesis and Cancer Program, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, 2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, VIC, Australia, 3 Department of Anatomy and Neuroscience, University of Melbourne, Melbourne, VIC, Australia The formation of the vertebrate vasculature involves the acquisition of endothelial cell identities, sprouting, migration, remodeling and maturation of functional vessel networks. To understand the cellular and molecular processes that drive vascular development, live-imaging of dynamic cellular events in the zebrafish embryo have proven highly informative. This review focusses on recent advances, new tools and new insights from imaging studies in vascular cell biology using zebrafish as a model system. Keywords: vasculogenesis, angiogenesis, lymphangiogenesis, anastomosis, endothelial cell, zebrafish Edited by: INTRODUCTION Elizabeth Anne Vincent Jones, KU Leuven, Belgium Vascular development is an early and essential process in the formation of a viable vertebrate Reviewed by: embryo. Blood and lymphatic vascular networks are composed of a complex mix of cell types: for Anna Rita Cantelmo, example smooth muscle cells, fibroblasts, pericytes and immune cells are intimately associated and Université Lille Nord de France, even integrated within mature vessel walls (Rouget, 1873; Horstmann, 1952; Nicosia and Madri, France 1987; Fantin et al., 2010; Gordon et al., 2010). The inner lining of the vasculature, made up of Jingjing Zhang, endothelial cells (ECs), is the earliest part of the vasculature to develop in the embryo and is Affiliated Hospital of Guangdong Medical University, China instructive in recruiting other lineages and cell types as the vascular system matures. -

Selective Venous Sampling for Primary Hyperparathyroidism: How to Perform an Examination and Interpret the Results with Reference to Thyroid Vein Anatomy
Jpn J Radiol DOI 10.1007/s11604-017-0658-3 INVITED REVIEW Selective venous sampling for primary hyperparathyroidism: how to perform an examination and interpret the results with reference to thyroid vein anatomy Takayuki Yamada1 · Masaya Ikuno1 · Yasumoto Shinjo1 · Atsushi Hiroishi1 · Shoichiro Matsushita1 · Tsuyoshi Morimoto1 · Reiko Kumano1 · Kunihiro Yagihashi1 · Takuyuki Katabami2 Received: 11 April 2017 / Accepted: 28 May 2017 © Japan Radiological Society 2017 Abstract Primary hyperparathyroidism (pHPT) causes and brachiocephalic veins for catheterization of the thyroid hypercalcemia. The treatment for pHPT is surgical dis- veins and venous anastomoses. section of the hyperfunctioning parathyroid gland. Lower rates of hypocalcemia and recurrent laryngeal nerve injury Keywords Primary hyperparathyroidism · Localization · imply that minimally invasive parathyroidectomy (MIP) is Thyroid vein · Venous sampling safer than bilateral neck resection. Current trends in MIP use can be inferred only by reference to preoperative locali- zation studies. Noninvasive imaging studies (typically pre- Introduction operative localization studies) show good detection rates of hyperfunctioning glands; however, there have also been Primary hyperparathyroidism (pHPT) is a common endocrine cases of nonlocalization or discordant results. Selective disease. Most patients have one adenoma, but double adeno- venous sampling (SVS) is an invasive localization method mas have been reported in up to 15% of cases [1]. Approxi- for detecting elevated intact parathyroid -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Normal Flow Signal of the Pterygoid Plexus on 3T MRA in Patients Without DAVF of the Cavernous Sinus
ORIGINAL RESEARCH EXTRACRANIAL VASCULAR Normal Flow Signal of the Pterygoid Plexus on 3T MRA in Patients without DAVF of the Cavernous Sinus K. Watanabe, S. Kakeda, R. Watanabe, N. Ohnari, and Y. Korogi ABSTRACT BACKGROUND AND PURPOSE: Cavernous sinuses and draining dural sinuses or veins are often visualized on 3D TOF MRA images in patients with dural arteriovenous fistulas involving the CS. Flow signals may be seen in the jugular vein and dural sinuses at the skull base on MRA images in healthy participants, however, because of reverse flow. Our purpose was to investigate the prevalence of flow signals in the pterygoid plexus and CS on 3T MRA images in a cohort of participants without DAVFs. MATERIALS AND METHODS: Two radiologists evaluated the flow signals of the PP and CS on 3T MRA images obtained from 406 consecutive participants by using a 5-point scale. In addition, the findings on 3T MRA images were compared with those on digital subtraction angiography images in an additional 171 participants who underwent both examinations. RESULTS: The radiologists identified 110 participants (27.1%; 108 left, 10 right, 8 bilateral) with evidence of flow signals in the PP alone (n ϭ 67) or in both the PP and CS (n ϭ 43). Flow signals were significantly more common in the left PP than in the right PP. In 171 patients who underwent both MRA and DSA, the MRA images showed flow signals in the PP with or without CS in 60 patients; no DAVFs were identified on DSA in any of these patients. CONCLUSIONS: Flow signals are frequently seen in the left PP on 3T MRA images in healthy participants. -

Papillary Thyroid Carcinoma
CASE REPORT Papillary Thyroid Carcinoma: The First Case of Direct Tumor Extension into the Left Innominate Vein Managed with a Single Operative Approach Douglas J Chung1, Diane Krieger2, Niberto Moreno3, Andrew Renshaw4, Rafael Alonso5, Robert Cava6, Mark Witkind7, Robert Udelsman8 ABSTRACT Aim: The aim of this study is to report a case of papillary thyroid carcinoma (PTC) with direct intravascular extension into the left internal jugular vein, resulting in tumor thrombus into the left innominate vein. Background: PTC is the most common of the four histological subtypes of thyroid malignancies,1 but PTC with vascular invasion into major blood vessels is rare.2 The incidence of PTC tumor thrombi was found to be 0.116% in one study investigating 7,754 thyroid surgical patients, and, of these patients with tumor thrombus, none extended more distal than the internal jugular vein.3 Koike et al.4 described a case of PTC invasion into the left innominate vein that was managed by a two-stage operative approach. Case description: A 58-year-old male presented with a rapidly growing left thyroid mass. Fine needle aspiration cytology (FNAC) suggested PTC and surgical exploration confirmed tumor extension into the left internal jugular vein. Continued dissection revealed a large palpable intraluminal tumor thrombus extending below the clavicle into the mediastinum, necessitating median sternotomy. Conclusion: Aggressive one-stage surgical resection resulted in successful en bloc extirpation of the tumor, with negative margins. Follow-up at 22 months postoperatively demonstrated no evidence of recurrence. Clinical significance: This is the first case of PTC extension into the left innominate vein managed with one-stage surgical intervention with curative intent. -
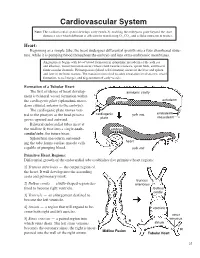
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision.