National Diabetes Registry Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Remembering the Dearly Departed
www.ipohecho.com.my IPOH echoechoYour Community Newspaper FREE for collection from our office and selected outlets, on 1st & 16th of the month. 30 sen for delivery to your ISSUE JULY 1 - 16, 2009 PP 14252/10/2009(022651) house by news vendors within Perak. RM 1 prepaid postage for mailing within Malaysia, Singapore and Brunei. 77 NEWS NEW! Meander With Mindy and discover what’s new in different sections of Ipoh A SOCIETY IS IPOH READY FOR HIDDEN GEMS TO EMPOWER THE INTERNATIONAL OF IPOH MALAYS TOURIST? 3 GARDEN SOUTH 11 12 REMEMBERING THE DEARLY DEPARTED by FATHOL ZAMAN BUKHARI The Kamunting Christian Cemetery holds a record of sorts. It has the largest number of Australian servicemen and family members buried in Malaysia. All in all 65 members of the Australian Defence Forces were buried in graves all over the country. Out of this, 40 were interred at the Kamunting burial site, which is located next to the Taiping Tesco Hypermarket. They were casualties of the Malayan Emergency (1948 to 1960) and Con- frontation with Indonesia (1963 to 1966). continued on page 2 2 IPOH ECHO JULY 1 - 16, 2009 Your Community Newspaper A fitting service for the Aussie soldiers who gave their lives for our country or over two decades headstones. Members, Ffamilies and friends their families and guests of the fallen heroes have then adjourned to the been coming regularly Taiping New Club for to Ipoh and Taiping to refreshments. honour their loved ones. Some come on their own Busy Week for Veterans while others make their The veterans made journey in June to coincide full use of their one-week with the annual memorial stay in Ipoh by attending service at the God’s Little other memorial services Acre in Batu Gajah. -

Combatting Pseudoscience: a Science Editorial and Health Literacy Workshop to Improve Scientific Literacy in 16-Year-Old Students in Malaysia
Combatting Pseudoscience: A Science Editorial and Health Literacy Workshop to Improve Scientific Literacy in 16-Year-Old Students in Malaysia Hannah M NAZRI Nuffield Department of Women’s & Reproductive Health, University of Oxford, Women’s Centre, John Radcliffe Hospital, United Kingdom Submitted: 11 Aug 2019 Accepted: 23 Sep 2019 Online: 4 Nov 2019 To cite this article: Nazri HM. Combatting pseudoscience: a science and health literacy workshop to improve scientific literacy in 16-year-old students in Malaysia.Malays J Med Sci. 2019;26(5):1–5. https://doi.org/10.21315/ mjms2019.26.5.1 To link to this article: https://doi.org/10.21315/mjms2019.26.5.1 Abstract The rise of dubious medical practice and anti-vaccination groups in Malaysia suggests that the public needs to be equipped with the scientific literacy skills to navigate the healthcare landscape. Additionally, the overall result of the Programme for International Student Assessment (PISA) 2009+ for Malaysia suggests that the national scientific literacy levels of 16-year-old Malaysian students to be below the international and the Organisation for Economic Co-operation and Development (OECD) average. Consequently, the Higher Order Thinking Skills (HOTS) was introduced to form part of the national English language evaluation in 2013 to encourage creative and critical thinking. In this editorial piece, I describe a youth-led intervention that may be more effective at increasing scientific literacy to combat pseudoscience in Malaysian youth especially in bridging the education inequality gap in Malaysia. Keywords: critical thinking, education, health literacy, health advocacy, misinformation, Malaysia Introduction specialist, may not be able to comment on stem cell therapy, for example. -

Soalan No : 93 Pemberitahuan Pertanyaan Dewan Rakyat Mesyuarat Ketiga, Penggal Ketiga Parlimen Keempat Belas Pertanyaan : Bukan
SOALAN NO : 93 PEMBERITAHUAN PERTANYAAN DEWAN RAKYAT MESYUARAT KETIGA, PENGGAL KETIGA PARLIMEN KEEMPAT BELAS PERTANYAAN : BUKAN LISAN DARIPADA : TUAN CHA KEE CHIN [RASAH] SOALAN TUAN CHA KEE CHIN [ RASAH ] minta MENTERI KESIHATAN menyatakan senarai jumlah pesakit positif COVID-19 dan kematian akibat jangkitan COVID-19 di Negeri Sembilan mengikut daerah dan mukim. JAWAPAN Tuan Yang di-Pertua, 1. Kementerian Kesihatan Malaysia (KKM) ingin memaklumkan sehingga 29 November 2020, kumulatif kes positif COVID-19 yang dilaporkan di Malaysia adalah sebanyak 64,485 kes. Daripada jumlah tersebut, Negeri Sembilan mencatatkan sebanyak 4,774 kes (7.4% daripada keseluruhan kes di Malaysia). Di Negeri Sembilan, kebanyakan kes dilaporkan dari daerah Seremban dan mukim Ampangan. Perincian jumlah kes mengikut daerah dan mukim Negeri Sembilan adalah Seremban (3,973 kes), Rembau (416 kes), Port Dickson (275 kes), Kuala Pilah (34 kes), Tampin (33 kes), Jempol (27 kes) dan Jelebu (16 kes). 2. Pecahan mengikut mukim bagi daerah Seremban adalah seperti berikut: i. Ampangan 1,432 kes; ii. Labu 1,133 kes; iii. Seremban 600 kes; iv. Rantau 358 kes; v. Rasah 277 kes; vi. Setul 151 kes; SOALAN NO : 93 vii. Lenggeng 22 kes; dan viii. Pantai 0 kes. 3. Pecahan mengikut mukim bagi daerah Port Dickson adalah seperti berikut: i. Jimah 110 kes; ii. Si Rusa 135 kes; iii. Port Dickson 19 kes; iv. Linggi 7 kes; dan v. Pasir Panjang 4 kes. 4. Pecahan mengikut mukim bagi daerah Jempol adalah seperti berikut: i. Rompin 8 kes; ii. Kuala jempol 6 kes; iii. Serting Ilir 6 kes; iv. Jelai 5 kes; dan v. Serting ulu 2 kes. -

Negeri Ppd Kod Sekolah Nama Sekolah Alamat Bandar Poskod Telefon Fax Negeri Sembilan Ppd Jempol/Jelebu Nea0025 Smk Dato' Undang
SENARAI SEKOLAH MENENGAH NEGERI SEMBILAN KOD NEGERI PPD NAMA SEKOLAH ALAMAT BANDAR POSKOD TELEFON FAX SEKOLAH PPD NEGERI SEMBILAN NEA0025 SMK DATO' UNDANG MUSA AL-HAJ KM 2, JALAN PERTANG, KUALA KLAWANG JELEBU 71600 066136225 066138161 JEMPOL/JELEBU PPD SMK DATO' UNDANG SYED ALI AL-JUFRI, NEGERI SEMBILAN NEA0026 BT 4 1/2 PERADONG SIMPANG GELAMI KUALA KLAWANG 71600 066136895 066138318 JEMPOL/JELEBU SIMPANG GELAMI PPD NEGERI SEMBILAN NEA6001 SMK BAHAU KM 3, JALAN ROMPIN BAHAU 72100 064541232 064542549 JEMPOL/JELEBU PPD NEGERI SEMBILAN NEA6002 SMK (FELDA) PASOH 2 FELDA PASOH 2 SIMPANG PERTANG 72300 064961185 064962400 JEMPOL/JELEBU PPD NEGERI SEMBILAN NEA6003 SMK SERI PERPATIH PUSAT BANDAR PALONG 4,5 & 6, GEMAS 73430 064666362 064665711 JEMPOL/JELEBU PPD NEGERI SEMBILAN NEA6005 SMK (FELDA) PALONG DUA FELDA PALONG 2 GEMAS 73450 064631314 064631173 JEMPOL/JELEBU PPD BANDAR SERI NEGERI SEMBILAN NEA6006 SMK (FELDA) LUI BARAT BANDAR SERI JEMPOL 72120 064676300 064676296 JEMPOL/JELEBU JEMPOL PPD NEGERI SEMBILAN NEA6007 SMK (FELDA) PALONG 7 FELDA PALONG TUJUH GEMAS 73470 064645464 064645588 JEMPOL/JELEBU PPD BANDAR SERI NEGERI SEMBILAN NEA6008 SMK (FELDA) BANDAR BARU SERTING BANDAR SERI JEMPOL 72120 064581849 064583115 JEMPOL/JELEBU JEMPOL PPD BANDAR SERI NEGERI SEMBILAN NEA6009 SMK SERTING HILIR KOMPLEKS FELDA SERTING HILIR 4 72120 064684504 064683165 JEMPOL/JELEBU JEMPOL PPD NEGERI SEMBILAN NEA6010 SMK PALONG SEBELAS (FELDA) FELDA PALONG SEBELAS GEMAS 73430 064669751 064669751 JEMPOL/JELEBU PPD BANDAR SERI NEGERI SEMBILAN NEA6011 SMK SERI JEMPOL -

Recycling in Ipoh:Beyond The
www.ipohecho.com.my FREE COPY IPOH Your Voiceechoecho In The Community Since 2006 June 1 - 15, 2017 PP 14252/10/2012(031136) 30 SEN FOR DELIVERY TO YOUR DOORSTEP – ISSUE ASK YOUR NEWSVENDOR 259 100,000 print readers 1,346,573 online hits in April (verifiable) Recycling in Ipoh: Beyond the Bin By Tan Mei Kuan he term “recycle” is defined by the Oxford dictionary as “to convert waste into reusable material”. TIpoh Echo zooms in on how non-governmental organisations and business entities here are going beyond the bin in getting the community and workforce to embrace the green culture. This is where every single one of us come in to play our part. Besides sustaining Mother Nature, recycling now enables us to provide for Continued on page 2 the disadvantaged in the community and also fund local charities via opportunity shops. All the more reason for us to recycle! Continued on page 2 KOHIJAU: Dr Richard (forth from right) with volunteers KOHIJAU: Cleanliness campaign during Thaipusam Anning Berhad: Sorting out papers Recycle bins at Meru Valley Resort Anning Berhad: Browsing pre-loved goods KOHIJAU-ICYCLE bin at Teluk Intan 2 June 1 - 15, 2017 IPOH ECHO Your Voice In The Community Take Recycling to Next Level – at Home, at Work and in Your Community Koperasi Alam Hijau Perak Berhad (KOHIJAU) operasi Alam Hijau Perak Berhad (KOHIJAU) is a co-operative formed by the NGO, KIpoh City Watch (ICW) in April 2016 to provide a solution to waste management through its high technology KOHIJAU-ICYCLE recycling system. The KOHIJAU-ICYCLE Recycling Reward Point System enables one to recycle for a sustainable environment and at the same time collect points to be exchanged for cash, shopping coupons or donated to welfare bodies. -

Buku Daftar Senarai Nama Jurunikah Kawasan-Kawasan Jurunikah Daerah Johor Bahru Untuk Tempoh 3 Tahun (1 Januari 2016 – 31 Disember 2018)
BUKU DAFTAR SENARAI NAMA JURUNIKAH KAWASAN-KAWASAN JURUNIKAH DAERAH JOHOR BAHRU UNTUK TEMPOH 3 TAHUN (1 JANUARI 2016 – 31 DISEMBER 2018) NAMA JURUNIKAH BI NO KAD PENGENALAN MUKIM KAWASAN L NO TELEFON 1 UST. HAJI MUSA BIN MUDA (710601-01-5539) 019-7545224 BANDAR -Pejabat Kadi Daerah Johor Bahru (ZON 1) 2 UST. FAKHRURAZI BIN YUSOF (791019-01-5805) 013-7270419 3 DATO’ HAJI MAHAT BIN BANDAR -Kg. Tarom -Tmn. Bkt. Saujana MD SAID (ZON 2) -Kg. Bahru -Tmn. Imigresen (360322-01-5539) -Kg. Nong Chik -Tmn. Bakti 07-2240567 -Kg. Mahmodiah -Pangsapuri Sri Murni 019-7254548 -Kg. Mohd Amin -Jln. Petri -Kg. Ngee Heng -Jln. Abd Rahman Andak -Tmn. Nong Chik -Jln. Serama -Tmn. Kolam Air -Menara Tabung Haji -Kolam Air -Dewan Jubli Intan -Jln. Straits View -Jln. Air Molek 4 UST. MOHD SHUKRI BIN BANDAR -Kg. Kurnia -Tmn. Melodies BACHOK (ZON 3) -Kg. Wadi Hana -Tmn. Kebun Teh (780825-01-5275) -Tmn. Perbadanan Islam -Tmn. Century 012-7601408 -Tmn. Suria 5 UST. AYUB BIN YUSOF BANDAR -Kg. Melayu Majidee -Flat Stulang (771228-01-6697) (ZON 4) -Kg. Stulang Baru 017-7286801 1 NAMA JURUNIKAH BI NO KAD PENGENALAN MUKIM KAWASAN L NO TELEFON 6 UST. MOHAMAD BANDAR - Kg. Dato’ Onn Jaafar -Kondo Datin Halimah IZUDDIN BIN HASSAN (ZON 5) - Kg. Aman -Flat Serantau Baru (760601-14-5339) - Kg. Sri Paya -Rumah Pangsa Larkin 013-3352230 - Kg. Kastam -Tmn. Larkin Perdana - Kg. Larkin Jaya -Tmn. Dato’ Onn - Kg. Ungku Mohsin 7 UST. HAJI ABU BAKAR BANDAR -Bandar Baru Uda -Polis Marin BIN WATAK (ZON 6) -Tmn. Skudai Kanan -Kg. -

No 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Taiping 15 16 17 NEGERI PERAK
NEGERI PERAK SENARAI TAPAK BEROPERASI : 17 TAPAK Tahap Tapak No Kawasan PBT Nama Tapak Alamat Tapak (Operasi) 1 Batu Gajah TP Batu Gajah Batu 3, Jln Tanjung Tualang, Batu Gajah Bukan Sanitari Jalan Air Ganda Gerik, Perak, 2 Gerik TP Jln Air Ganda Gerik Bukan Sanitari D/A MDG 33300 Gerik, Perak Batu. 8, Jalan Bercham, Tanjung 3 Ipoh TP Bercham Bukan Sanitari Rambutan, Ipoh, Perak Batu 21/2, Jln. Kuala Dipang, Sg. Siput 4 Kampar TP Sg Siput Selatan Bukan Sanitari (S), Kampar, Perak Lot 2720, Permatang Pasir, Alor Pongsu, 5 Kerian TP Bagan Serai Bukan Sanitari Beriah, Bagan Serai KM 8, Jalan Kuala Kangsar, Salak Utara, 6 Kuala Kangsar TP Jln Kuala Kangsar Bukan Sanitari Sungai Siput 7 Lenggong TP Ayer Kala Lot 7345 & 7350, Ayer Kala, Lenggong Bukan Sanitari Batu 1 1/2, Jalan Beruas - Sitiawan, 8 Manjung TP Sg Wangi Bukan Sanitari 32000 Sitiawan 9 Manjung TP Teluk Cempedak Teluk Cempedak, Pulau Pangkor Bukan Sanitari 10 Manjung TP Beruas Kg. Che Puteh, Jalan Beruas - Taiping Bukan Sanitari Bukit Buluh, Jalan Kelian Intan, 33100 11 Pengkalan Hulu TP Jln Gerik Bukan Sanitari Pengkalan Hulu 12 Perak Tengah TP Parit Jln Chopin Kanan, Parit Bukan Sanitari 13 Selama TP Jln Tmn Merdeka Kg. Lampin, Jln. Taman Merdeka, Selama Bukan Sanitari Lot 1706, Mukim Jebong, Daerah Larut 14 Taiping TP Jebong Bukan Sanitari Matang dan Selama Kampung Penderas, Slim River, Tanjung 15 Tanjung Malim TP Penderas Bukan Sanitari Malim 16 Tapah TP Bidor, Pekan Pasir Kampung Baru, Pekan Pasir, Bidor Bukan Sanitari 17 Teluk Intan TP Changkat Jong Batu 8, Jln. -

Senarai Bilangan Pemilih Mengikut Dm Sebelum Persempadanan 2016 Johor
SURUHANJAYA PILIHAN RAYA MALAYSIA SENARAI BILANGAN PEMILIH MENGIKUT DAERAH MENGUNDI SEBELUM PERSEMPADANAN 2016 NEGERI : JOHOR SENARAI BILANGAN PEMILIH MENGIKUT DAERAH MENGUNDI SEBELUM PERSEMPADANAN 2016 NEGERI : JOHOR BAHAGIAN PILIHAN RAYA PERSEKUTUAN : SEGAMAT BAHAGIAN PILIHAN RAYA NEGERI : BULOH KASAP KOD BAHAGIAN PILIHAN RAYA NEGERI : 140/01 SENARAI DAERAH MENGUNDI DAERAH MENGUNDI BILANGAN PEMILIH 140/01/01 MENSUDOT LAMA 398 140/01/02 BALAI BADANG 598 140/01/03 PALONG TIMOR 3,793 140/01/04 SEPANG LOI 722 140/01/05 MENSUDOT PINDAH 478 140/01/06 AWAT 425 140/01/07 PEKAN GEMAS BAHRU 2,391 140/01/08 GOMALI 392 140/01/09 TAMBANG 317 140/01/10 PAYA LANG 892 140/01/11 LADANG SUNGAI MUAR 452 140/01/12 KUALA PAYA 807 140/01/13 BANDAR BULOH KASAP UTARA 844 140/01/14 BANDAR BULOH KASAP SELATAN 1,879 140/01/15 BULOH KASAP 3,453 140/01/16 GELANG CHINCHIN 671 140/01/17 SEPINANG 560 JUMLAH PEMILIH 19,072 SENARAI BILANGAN PEMILIH MENGIKUT DAERAH MENGUNDI SEBELUM PERSEMPADANAN 2016 NEGERI : JOHOR BAHAGIAN PILIHAN RAYA PERSEKUTUAN : SEGAMAT BAHAGIAN PILIHAN RAYA NEGERI : JEMENTAH KOD BAHAGIAN PILIHAN RAYA NEGERI : 140/02 SENARAI DAERAH MENGUNDI DAERAH MENGUNDI BILANGAN PEMILIH 140/02/01 GEMAS BARU 248 140/02/02 FORTROSE 143 140/02/03 SUNGAI SENARUT 584 140/02/04 BANDAR BATU ANAM 2,743 140/02/05 BATU ANAM 1,437 140/02/06 BANDAN 421 140/02/07 WELCH 388 140/02/08 PAYA JAKAS 472 140/02/09 BANDAR JEMENTAH BARAT 3,486 140/02/10 BANDAR JEMENTAH TIMOR 2,719 140/02/11 BANDAR JEMENTAH TENGAH 414 140/02/12 BANDAR JEMENTAH SELATAN 865 140/02/13 JEMENTAH 365 140/02/14 -

Malaysian Communications and Multimedia Commission
MALAYSIAN COMMUNICATIONS AND MULTIMEDIA COMMISSION INVITATION TO REGISTER INTEREST AND SUBMIT A DRAFT UNIVERSAL SERVICE PLAN AS A UNIVERSAL SERVICE PROVIDER UNDER THE COMMUNICATIONS AND MULTIMEDIA (UNIVERSAL SERVICE PROVISION) REGULATIONS 2002 FOR THE INSTALLATION OF NETWORK FACILITIES AND DEPLOYMENT OF NETWORK SERVICE FOR THE PROVISIONING OF PUBLIC CELLULAR SERVICES AT THE UNIVERSAL SERVICE TARGETS UNDER THE JALINAN DIGITAL NEGARA (JENDELA) PHASE 1 INITIATIVE Ref: MCMC/USPD/PDUD(01)/JENDELA_P1/TC/11/2020(05) Date: 20 November 2020 Invitation to Register Interest as a Universal Service Provider MCMC/USPD/PDUD(01)/JENDELA_P1/TC/11/2020(05) Page 1 of 142 TABLE OF CONTENTS ABBREVIATIONS ............................................................................................................................. 4 INTERPRETATION ........................................................................................................................... 5 SECTION I – INTRODUCTION ........................................................................................................ 8 1. BACKGROUND ......................................................................................................................... 8 SECTION II – DESCRIPTION OF SCOPE OF WORK .............................................................. 10 2. GENERAL DESCRIPTION OF THE FACILITIES AND SERVICES TO BE PROVIDED ....................................................................................................................................... 10 3. SCOPE OF -

Malaysian Statistics on Medicines 2009 & 2010
MALAYSIAN STATISTICS ON MEDICINES 2009 & 2010 Edited by: Siti Fauziah A., Kamarudin A., Nik Nor Aklima N.O. With contributions from: Faridah Aryani MY., Fatimah AR., Sivasampu S., Rosliza L., Rosaida M.S., Kiew K.K., Tee H.P., Ooi B.P., Ooi E.T., Ghan S.L., Sugendiren S., Ang S.Y., Muhammad Radzi A.H. , Masni M., Muhammad Yazid J., Nurkhodrulnada M.L., Letchumanan G.R.R., Fuziah M.Z., Yong S.L., Mohamed Noor R., Daphne G., Chang K.M., Tan S.M., Sinari S., Lim Y.S., Tan H.J., Goh A.S., Wong S.P., Fong AYY., Zoriah A, Omar I., Amin AN., Lim CTY, Feisul Idzwan M., Azahari R., Khoo E.M., Bavanandan S., Sani Y., Wan Azman W.A., Yusoff M.R., Kasim S., Kong S.H., Haarathi C., Nirmala J., Sim K.H., Azura M.A., Suganthi T., Chan L.C., Choon S.E., Chang S.Y., Roshidah B., Ravindran J., Nik Mohd Nasri N.I, Wan Hamilton W.H., Zaridah S., Maisarah A.H., Rohan Malek J., Selvalingam S., Lei C.M., Hazimah H., Zanariah H., Hong Y.H.J., Chan Y.Y., Lin S.N., Sim L.H., Leong K.N., Norhayati N.H.S, Sameerah S.A.R, Rahela A.K., Yuzlina M.Y., Hafizah ZA ., Myat SK., Wan Nazuha W.R, Lim YS,Wong H.S., Rosnawati Y., Ong S.G., Mohd. Shahrir M.S., Hussein H., Mary S.C., Marzida M., Choo Y. M., Nadia A.R., Sapiah S., Mohd. Sufian A., Tan R.Y.L., Norsima Nazifah S., Nurul Faezah M.Y., Raymond A.A., Md. -
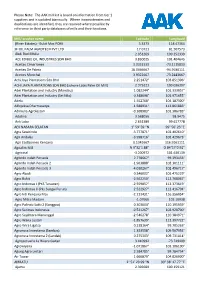
The AAK Mill List Is Based on Information from Tier 1 Suppliers and Is Updated Biannually
Please Note: The AAK mill list is based on information from tier 1 suppliers and is updated biannually. Where inconsistencies and duplications are identified, they are resolved where possible by reference to third party databases of mills and their locations. Mill/ crusher name Latitude Longitude (River Estates) - Bukit Mas POM 5.3373 118.47364 3F OIL PALM AGROTECH PVT LTD 17.0721 81.507573 Abdi Budi Mulia 2.051269 100.252339 ACE EDIBLE OIL INDUSTRIES SDN BHD 3.830025 101.404645 Aceites Cimarrones 3.0352333 -73.1115833 Aceites De Palma 18.0466667 -94.9186111 Aceites Morichal 3.9322667 -73.2443667 Achi Jaya Plantations Sdn Bhd 2.251472° 103.051306° ACHI JAYA PLANTATIONS SDN BHD (Johore Labis Palm Oil Mill) 2.375221 103.036397 Adei Plantation and Industry (Mandau) 1.082244° 101.333057° Adei Plantation and Industry (Sei Nilo) 0.348098° 101.971655° Adela 1.552768° 104.187300° Adhyaksa Dharmasatya -1.588931° 112.861883° Adimulia Agrolestari -0.108983° 101.386783° Adolina 3.568056 98.9475 Aek Loba 2.651389 99.617778 AEK NABARA SELATAN 1° 59' 59 "N 99° 56' 23 "E Agra Sawitindo -3.777871° 102.402610° Agri Andalas -3.998716° 102.429673° Agri Eastborneo Kencana 0.1341667 116.9161111 Agrialim Mill N 9°32´1.88" O 84°17´0.92" Agricinal -3.200972 101.630139 Agrindo Indah Persada 2.778667° 99.393433° Agrindo Indah Persada 2 -1.963888° 102.301111° Agrindo Indah Persada 3 -4.010267° 102.496717° Agro Abadi 0.346002° 101.475229° Agro Bukit -2.562250° 112.768067° Agro Indomas I (PKS Terawan) -2.559857° 112.373619° Agro Indomas II (Pks Sungai Purun) -2.522927° -

NEGERI SEMBILAN P = Parlimen / Parliament N = Dewan Undangan Negeri (DUN)
NEGERI SEMBILAN P = Parlimen / Parliament N = Dewan Undangan Negeri (DUN) KAWASAN / STATE PENYANDANG / INCUMBENT PARTI / PARTY P126 JELEBU ZAINUDIN BIN ISMAIL BN N12601 – CHENNAH LOKE SIEW FOOK DAP N12602 – PERTANG JALALUDDIN BIN ALIAS BN N12603 - SUNGAI LUI MOHD. RAZI BIN MOHD. ALI BN N12604 - KLAWANG YUNUS BIN RAHMAT BN P127 JEMPOL MOHD ISA BIN ABDUL SAMAD BN N12705 – SERTING SHAMSHULKAHAR MOHD DELI BN N12706 – PALONG LILAH BIN YASIN BN N12707 - JERAM PADANG MANICKAM A/L LETCHUMAN BN N12708 - BAHAU CHEW SEH YONG DAP P128 SEREMBAN LOKE SIEW FOOK DAP N12809 – LENGGENG ISHAK BIN ISMAIL BN N12810 – NILAI ARUL KUMAR A/L JAMBUNATHAN DAP N12811 – LOBAK SIOW KIM LEONG DAP N12812 – TEMIANG NG CHIN TSAI DAP N12813 – SIKAMAT AMINUDDIN BIN HARUN PKR N12814 - AMPANGAN ABU UBAIDAH BIN HAJI REDZA BN P129 KUALA PILAH HASAN BIN MALEK BN N12915 - JUASSEH MOHAMMAD RAZI BIN KAIL BN N12916 - SERI MENANTI ABD SAMAD BIN IBRAHIM BN N12917 – SENALING ISMAIL BIN LASIM BN N12918 – PILAH NORHAYATI BINTI OMAR BN N12919 - JOHOL ABU SAMAH BIN MAHAT BN P130 RASAH TEO KOK SEONG DAP N13020 – LABU HASIM BIN RUSDI BN N13021 - BUKIT KEPAYANG CHA KEE CHIN DAP N13022 – RAHANG MARRY JOSEPHINE PRITTAM SINGH DAP N13023 – MAMBAU YAP YEW WENG DAP N13024 - SENAWANG GUNASEKAREN A/L PALASAMY DAP P131 REMBAU KHAIRY JAMALUDDIN ABU BAKAR BN N13125 – PAROI MOHD GHAZALI BIN ABD WAHID BN N13126 – CHEMBONG ZAIFULBAHRI BIN IDRIS BN N13127 – RANTAU MOHAMAD BIN HAJI HASAN BN N13128 - KOTA AWALUDIN BIN SAID BN P132 TELOK KEMAN G KAMARUL BAHARIN BIN ABBAS PKR N13229 – CHUAH CHAI TONG CHAI PKR N13230 – LUKUT EAN YONG TIN SIN DAP N13231 - BAGAN PINANG TUN HAIRUDIN BIN ABU BAKAR BN N13232 – LINGGI ABDUL RAHMAN BIN MOHD REDZA BN N13233 - PORT DICKSON RAVI A/L MUNUSAMY PKR P133 T AMPIN SHAZIMAN BIN ABU MANSOR BN N13334 – GEMAS ABD RAZAK BIN AB SAID BN N13335 – GEMENCHEH MOHD ISAM BIN MOHD ISA BN N13336 - REPAH VEERAPAN A/L SUPERAMANIAM DAP .