Diversity in Host Preference of Rotylenchus Spp. Y.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Begonia Jinyunensis (Begoniaceae, Section Platycentrum), a New
Ding et al. Botanical Studies 2014, 55:62 http://www.as-botanicalstudies.com/content/55/1/62 RESEARCH Open Access Begonia jinyunensis (Begoniaceae, section Platycentrum), a new palmately compound leaved species from Chongqing, China Bo Ding1†, Koh Nakamura2*†, Yoshiko Kono2, Meng-Jung Ho2 and Ching-I Peng2* Abstract Background: Continental China is the center of Begonia species diversity in Asia and contains more than 60 species out of about 110 named species of section Platycentrum. Mt. Jinyun, located in Chongqing City at the upper reaches of the Yangtze River, harbors a subtropical broadleaved forest with high species diversity. During a botanical survey in Mt. Jinyun, an unknown Begonia species of sect. Platycentrum with palmately compound leaves was collected and studied based on detailed morphological observations and cytological and molecular phylogenetic analyses. Results: The unknown Begonia bears a superficial resemblance to B. hemsleyana in having palmately compound leaves, a feature unseen in other species of sect. Platycentrum in China. It is however sharply distinct from the latter in the acaulous habit with aerial stems seen only at anthesis and long rhizomes (vs. erect stems to 70 cm or taller with short rhizomes), 4–6 pinnatilobed leaflets with indistinct, decurrent petiolules (vs. 7–10 serrate leaflets with distinct petiolules), and white (vs. pink) tepals. Molecular phylogenetic analyses based on nuclear ribosomal DNA and chloroplast DNA sequences indicated that this species was allied to Platycentrum species occurring in Southwest and South-central China and Vietnam, including B. hemsleyana, and clearly separable from these species. Somatic chromosome number of 2n = 22 was reported for this unknown species. -
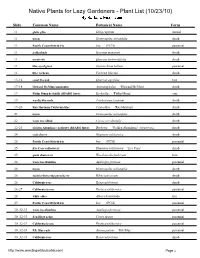
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

1 Retail Listings 2011 by USDA Zone, As of Sept 5 - Please Check for Current Availability
1 Retail listings 2011 by USDA zone, as of Sept 5 - please check for current availability USDA zone: 2 Alcea rosea 'Nigra' Classic hollyhock with dark maroon, nearly black flowers covering the 5-8 ft spires in July and August. They like well-drained soil and full to part sun with average summer water. Short-lived, they reseed easily establishing long-lived colonies. Frost hardy in USDA zone 2. 4in @ $3 Malvaceae Lindelofia longiflora Bright blue flowered cousin of a forget-me-not which blooms from late spring to frost. Long-live perennial, clumping to 2 ft by 2 ft in rich, moist soil in a half shady spot– think woodland. Great for a border that gets some water, but not much attention otherwise. Hardy to 25 below. 6in @ $12 Boraginaceae Physocarpus opulifolius 'Dart's Gold' golden ninebark Its golden foliage highlights the pure white, fragrant, summer flowers and brilliant red fruit in autumn. Peeling bark adds interest to this durable hedging plant or specimen, deciduous, to 5 ft tall and wide, smaller than the species. Out of the hottest afternoon sun seems to suit it best for foliage color. Can take a bit of drought, but best with a little summer water. Takes will to pruning. Frost hardy in USDA zone 2. 1g @ $12, 2g @ $22 Rosaceae Rosa glauca red leaf rose Grown as much for its foliage as its flowers this deciduous shrub, to 6 ft tall x 5 ft wide, has glaucous blue foliage and, in June, single pink flowers with white centers. Lovely rose hips follow and remain through the winter. -

Seed Ecology Iii
SEED ECOLOGY III The Third International Society for Seed Science Meeting on Seeds and the Environment “Seeds and Change” Conference Proceedings June 20 to June 24, 2010 Salt Lake City, Utah, USA Editors: R. Pendleton, S. Meyer, B. Schultz Proceedings of the Seed Ecology III Conference Preface Extended abstracts included in this proceedings will be made available online. Enquiries and requests for hardcopies of this volume should be sent to: Dr. Rosemary Pendleton USFS Rocky Mountain Research Station Albuquerque Forestry Sciences Laboratory 333 Broadway SE Suite 115 Albuquerque, New Mexico, USA 87102-3497 The extended abstracts in this proceedings were edited for clarity. Seed Ecology III logo designed by Bitsy Schultz. i June 2010, Salt Lake City, Utah Proceedings of the Seed Ecology III Conference Table of Contents Germination Ecology of Dry Sandy Grassland Species along a pH-Gradient Simulated by Different Aluminium Concentrations.....................................................................................................................1 M Abedi, M Bartelheimer, Ralph Krall and Peter Poschlod Induction and Release of Secondary Dormancy under Field Conditions in Bromus tectorum.......................2 PS Allen, SE Meyer, and K Foote Seedling Production for Purposes of Biodiversity Restoration in the Brazilian Cerrado Region Can Be Greatly Enhanced by Seed Pretreatments Derived from Seed Technology......................................................4 S Anese, GCM Soares, ACB Matos, DAB Pinto, EAA da Silva, and HWM Hilhorst -

BEGONIACEAE 1. BEGONIA Linnaeus, Sp. Pl. 2: 1056. 1753
BEGONIACEAE 秋海棠科 qiu hai tang ke Gu Cuizhi (谷粹芝 Ku Tsue-chih)1, Ching-I Peng (彭镜毅)2, Nicholas J. Turland3 Perennial succulent herbs, very rarely subshrubs. Stem erect, frequently rhizomatous, or plants tuberous and either acaulescent or shortly stemmed, rarely lianoid or climbing with adventitious roots, or stoloniferous. Leaves simple, rarely palmately compound, alternate or all basal, petiolate, stipules usually deciduous; blade often oblique and asymmetric, rarely symmetric, margin irregularly serrate and divided, occasionally entire, venation usually palmate. Flowers unisexual, plants monoecious, rarely dioecious, (1 or)2–4 to several, rarely numerous in dichotomous cyme, sometimes in panicles, with pedicel and bracts. Staminate flower: tepals 2 or 4 and decussate, usually outer ones larger, inner ones smaller; stamens usually numerous; filaments free or connate at base; anthers 2- celled, apical or lateral. Pistillate flower: tepals 2–5(–10), usually free, rarely connate at base; ovary nodding, pendulous, or ascending, 1–3-, rarely 4–8-loculed; placentae axile or parietal; styles 2 or 3(or more), free or fused at base, forked once or more; stigma turgid, spirally twisted-tortuous or U-shaped, capitate or reniform and setose-papillose. Capsule dry, sometimes berrylike, unequally or subequally 3-winged, rarely wingless and 3- or 4-horned; seeds very numerous, minute, oblong, testa pale brown, reticulate. Two or three genera and more than 1400 species: widely distributed in the tropical and subtropical regions of the world; one genus and 173 species (141 endemic) in China. Ku Tsuechih. 1999. Begoniaceae. In: Ku Tsuechih, ed., Fl. Reipubl. Popularis Sin. 52(1): 126–269. 1. -

2018–2019 Catalog
2018–2019 CATALOG W www.woodburnnursery.com S 888-634-2232 P 503-634-2231 F 503-634-2238 1 Catalog and Program Information Special Tags for Retail Customers: Information for special tags is due in our office by February 1st. If you require special tags with retail prices or other information, the sooner we know the better. Let your sales representative know you are interested so we can provide you with the proper paperwork. There is no additional charge for this service. Spring Billing Program: This program provides customers with the opportunity to ship material from September 1, 2018 through February 28, 2019 and delay payment until May 1, 2019. Requirements are as follows: 1| The first nursery stock order shipped must equal or exceed 4,000 units, where one unit is equivalent to a one gallon pot. 2| Once a customer ships an order meeting these requirements, any subsequent orders will also qualify if shipped on or before February 28th. 3| Payment is due in our office on or before May 1, 2019. Those customers that miss this date are not eligible for the program the following season. Prices listed in this catalog are subject to change due to minimum wage increases. Claims: No claim will be honored unless made in writing on the bill of lading upon receipt of the shipment. We also request notification within 5 days of delivery if there is a count discrepancy or if any damage occurred during shipment. It will be at the sole discretion of Woodburn Nursery and Azaleas, Inc. to evaluate and issue any and all credit adjustments. -

Conservation Management Zones of Australia
Conservation Management Zones of Australia Mitchell Grasslands Prepared by the Department of the Environment Acknowledgements This project and its associated products are the result of collaboration between the Department of the Environment’s Biodiversity Conservation Division and the Environmental Resources Information Network (ERIN). Invaluable input, advice and support were provided by staff and leading researchers from across the Department of Environment (DotE), Department of Agriculture (DoA), the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the academic community. We would particularly like to thank staff within the Wildlife, Heritage and Marine Division, Parks Australia and the Environment Assessment and Compliance Division of DotE; Nyree Stenekes and Robert Kancans (DoA), Sue McIntyre (CSIRO), Richard Hobbs (University of Western Australia), Michael Hutchinson (ANU); David Lindenmayer and Emma Burns (ANU); and Gilly Llewellyn, Martin Taylor and other staff from the World Wildlife Fund for their generosity and advice. Special thanks to CSIRO researchers Kristen Williams and Simon Ferrier whose modelling of biodiversity patterns underpinned identification of the Conservation Management Zones of Australia. Image Credits Front Cover: Lawn Hill National Park – Peter Lik Page 4: Kowaris (Dasyuroides byrnei) – Leong Lim Page 10: Oriental Pratincole (Glareola maldivarum) – JJ Harrison Page 16: Australian Fossil Mammal Sites (Riversleigh) – World Heritage Listed site – Colin Totterdell Page 18: Mitchell Grasslands -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

A Note on Host Diversity of Criconemaspp
280 Pantnagar Journal of Research [Vol. 17(3), September-December, 2019] Short Communication A note on host diversity of Criconema spp. Y.S. RATHORE ICAR- Indian Institute of Pulses Research, Kanpur- 208 024 (U.P.) Key words: Criconema, host diversity, host range, Nematode Nematode species of the genus Criconema (Tylenchida: showed preference over monocots. Superrosids and Criconemitidae) are widely distributed and parasitize Superasterids were represented by a few host plants only. many plant species from very primitive orders to However, Magnoliids and Gymnosperms substantially advanced ones. They are migratory ectoparasites and feed contributed in the host range of this nematode species. on root tips or along more mature roots. Reports like Though Rosids revealed greater preference over Asterids, Rathore and Ali (2014) and Rathore (2017) reveal that the percent host families and orders were similar in number most nematode species prefer feeding on plants of certain as reflected by similar SAI values. The SAI value was taxonomic group (s). In the present study an attempt has slightly higher for monocots that indicate stronger affinity. been made to precisely trace the host plant affinity of The same was higher for gymnosperms (0.467) in twenty-five Criconema species feeding on diverse plant comparison to Magnolids (0.413) (Table 1). species. Host species of various Criconema species Perusal of taxonomic position of host species in Table 2 reported by Nemaplex (2018) and others in literature were revealed that 68 % of Criconema spp. were monophagous aligned with families and orders following the modern and strictly fed on one host species. Of these, 20 % from system of classification, i.e., APG IV system (2016). -

Recent Changes in the Names of New Zealand Tree and Shrub Species
-- -- - Recent changes in the names of New Zealand tree and shrub species - Since the publication of 'Flora of New Zealand' Volume 1 (A- iii) Podocarpus dacydioides Dacrycarpus ducydioides lan 1961),covering indigenous ferns, conifers and dicots, there (iii)Podocarpus ferrugzneus Prumnopitys ferruginea have been major advances in taxonomic research and the clas- Podocarpus spicatus Prumnopitys taxijolia sification of many plant groups revised accordingly. Most of (iv1 Dacrydium cupressinum (unchanged) these changes have been summarised in the Nomina Nova (v)Dacrydium bidwillii Halocarpus bidwillii series published in the New Zealand Journal of Botany (Edgar Dacrydium bijorme Halocarpus bijormis 1971, Edgar and Connor 1978, 1983) and are included in re- Dacrydium kirkii Halocarpus kirkii cent books on New Zealand plants ie.g. Eagle 1982, Wilson (vi)Dacydium colensoi Lagarostrobos colensoi 1982). A number of these name changes affect important (vii)Dacrydium intermediurn Lepidothamnus intermedius forest plants and as several of these new names are now start- Dacrydium laxijolium Lepidotbamnus laxijolius ing to appear in the scientific literature, a list of changes af- (viii)Phyllocladus trichomanoidi~(unchanged) fecting tree and shrub taxa are given here. As a large number Phyllocladus glaucus (unchanged) of the readers of New Zealand Forestry are likely to use Poole Phyllocladus alpinus Phyllocladus aspleniijolius and Adams' "Trees and Shrubs of New Zealand" as their var. alpinus* * main reference for New Zealand forest plants, all the name changes are related to the fourth impression of this book. * It has been suggested that the Colenso name P, cunnin- it is important to realise that not all botanists necessarily ghamii (1884)should take precedence over the later (18891 ark agree with one particular name and you are not obliged to use name (P. -

Senex Energy Ltd
Senex Energy Ltd Project Atlas EPBC Referral – Water Report Volume 4 Appendix II D10171A04 October 2018 Senex Energy Ltd Water Report Project Atlas – EPBC Referral Final APPENDIX II Terrestrial GDEs Report 181030R_EPBC Water Report.docx D10171A04 October 2018 Client name Project Month 2015 Senex Project Atlas Terrestrial GDE Assessment Report Wandoan, Queensland Report prepared for Hydrobiology July 2018 1807_AUSEC_Hydrobiology_SenexAtlas_GDEReport_Rev2.docx P a g e | i Hydrobiology Senex Project Atlas Terrestrial GDE Assessment July 2018 This document has been prepared and is certified by: AUSECOLOGY PTY LTD ABN 15 155 304 751 PO Box 594, Morningside, QLD 4170 w www.ausecology.com e [email protected] Document status Revision Reason for issue Author Reviewed Issued to Date A Internal Draft Rohan Etherington Ralf Regeer - 08/08/2018 Hydrobiology 0 Issued to client Rohan Etherington Ralf Regeer 09/08/2018 KCB Revised from 1 Rohan Etherington Ralf Regeer Senex 28/08/2018 KCB comments Revised from 2 Rohan Etherington Ralf Regeer Senex 04/10/2018 Senex comments 1807_AUSEC_Hydrobiology_SenexAtlas_GDEReport_Rev2.docx P a g e | i Hydrobiology Senex Project Atlas Terrestrial GDE Assessment July 2018 Table of contents Glossary of Terms and Acronyms ______________________________________________________________ iv Executive Summary __________________________________________________________________________ v 1 Introduction ___________________________________________________________________________ 1 Background ____________________________________________________________________________