Data Elements and Definitions (Heart Failure)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2.3. Heart Sound and Auscultation
Dinesh Kumar Dinesh Dinesh Kumar CARDIOVASCULAR DISEASE ASSESSMENT DISEASE CARDIOVASCULAR AUTOMATIC HEART FOR SOUND AUTOMATIC ANALYSIS AUTOMATIC HEART SOUND ANALYSIS FOR CARDIOVASCULAR DISEASE ASSESSMENT Doctoral thesis submitted to the Doctoral Program in Information Science and Technology, supervised by Prof. Dr. Paulo Fernando Pereira de Carvalho and Prof. Dr. Manuel de Jesus Antunes, and presented to the Department of Informatics Engineering of the Faculty of Sciences and Technology of the University of Coimbra. September 2014 OIMBRA C E D NIVERSIDADE NIVERSIDADE U September 2014 Thesis submitted to the University of Coimbra in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Information Science and Technology This work was carried out under the supervision of Professor Paulo Fernando Pereira de Carvalho Professor Associado do Departamento de Engenharia Informática da Faculdade de Ciências e Tecnologia da Universidade de Coimbra and Professor Doutor Manuel J Antunes Professor Catedrático da Faculdade de Medicina da Universidade de Coimbra ABSTRACT Cardiovascular diseases (CVDs) are the most deadly diseases worldwide leaving behind diabetes and cancer. Being connected to ageing population above 65 years is prone to CVDs; hence a new trend of healthcare is emerging focusing on preventive health care in order to reduce the number of hospital visits and to enable home care. Auscultation has been open of the oldest and cheapest techniques to examine the heart. Furthermore, the recent advancement in digital technology stethoscopes is renewing the interest in auscultation as a diagnosis tool, namely for applications for the homecare context. A computer-based auscultation opens new possibilities in health management by enabling assessment of the mechanical status of the heart by using inexpensive and non-invasive methods. -

Very Important Extra Information
Very important Extra information * Guyton corners, anythingthat is colored with grey is EXTRA explanation Heart sounds &Murmurs Objectives : • Enumerate the different heart sounds. • Describe the cause and characteristic features of first and second heart sounds. • Correlate the heart sounds with different phases of cardiac cycle. • Define murmurs and their clinical importance. 2 Contact us : [email protected] Areas of Auscultation • Guyton corner : ( Normal Heart Sounds ) : Listening with a stethoscope to a normal heart, onehears a sound usually described as “lub, dub, lub, dub.”The “lub”is associated with closure of the atrioventricular (A-V) valves at the beginning of systole, and the “dub”is associated with closure of the semilunar (aortic and pulmonary) valves at the end of systole. The “lub” sound is called the first heart sound, and the “dub”is called the second heart sound, because the normal pumping cycle of the heart is considered to start when the A-V valves close at the onset of ventricular systole. 3 Heart Sounds There are four heart sounds SI, S2, S3 & S4. Two heart sound are audible with stethoscope S1&S2 (Lub - Dub). S3 &S4 are not audible with stethoscope Under normal conditions because they are low frequency sounds. Ventricular Systole is between First and second Heart sound. Ventricular diastole is between Second and First heart sounds. 4 Heart Sounds • Guyton corner : Phonocardiogram If a microphone specially designed to detect low-frequency sound is placed on the chest, the heart sounds can be amplified and recorded by a high-speed recording apparatus. The recording is called a phonocardiogram, and the heart sounds appear as waves, as shown schematically . -

An Audio Guide to Pediatric and Adult Heart Murmurs
Listen Up! An Audio Guide to Pediatric and Adult Heart Murmurs May 9, 2018 Dr. Michael Grattan Dr. Andrew Thain https://pollev.com/michaelgratt679 Case • You are working at an urgent care centre when a 40 year old recent immigrant from Syria presents with breathlessness. • You hear the following on cardiac auscultation: • What do you hear? • How can you describe what you hear so another practitioner will understand exactly what you mean? • What other important information will help you determine the significance of your auscultation? Objectives • In pediatric and adult patients: – To provide a general approach to cardiac auscultation – To review the most common pathologic and innocent heart murmurs • To emphasize the importance of a thorough history and physical exam (in addition to murmur description) in determining underlying etiology for heart problems Outline • A little bit of physiology and hemodynamics (we promise not too much) • Interactive pediatric and adult cases – https://pollev.com/michaelgratt679 – Get your listening ears ready! • Systolic murmurs (pathologic and innocent) • Diastolic murmurs • Continuous murmurs • Some other stuff Normal Heart Sounds Normal First & Second Sounds Splitting of 2nd heart sound Physiological : • Venous return to right is increased in inspiration – causes delayed closure of the pulmonary valve. • Simultaneously, return to left heart is reduced - premature closure of the aortic valve. • Heart sounds are unsplit when the patient holds breath at end expiration. Fixed: • No alteration in splitting with respiration. • In a patient with ASD – In expiration there is reduced pressure in the right atrium and increased pressure in the left atrium. • Blood is shunted to the right and this delays closure of the pulmonary valve in the same way as would occur in inspiration. -

Ministry of Health of Ukraine Kharkiv National Medical University
Ministry of Health of Ukraine Kharkiv National Medical University AUSCULTATION OF THE HEART. NORMAL HEART SOUNDS, REDUPLICATION OF THE SOUNDS, ADDITIONAL SOUNDS (TRIPLE RHYTHM, GALLOP RHYTHM), ORGANIC AND FUNCTIONAL HEART MURMURS Methodical instructions for students Рекомендовано Ученым советом ХНМУ Протокол №__от_______2017 г. Kharkiv KhNMU 2017 Auscultation of the heart. normal heart sounds, reduplication of the sounds, additional sounds (triple rhythm, gallop rhythm), organic and functional heart murmurs / Authors: Т.V. Ashcheulova, O.M. Kovalyova, O.V. Honchar. – Kharkiv: KhNMU, 2017. – 20 с. Authors: Т.V. Ashcheulova O.M. Kovalyova O.V. Honchar AUSCULTATION OF THE HEART To understand the underlying mechanisms contributing to the cardiac tones formation, it is necessary to remember the sequence of myocardial and valvular action during the cardiac cycle. During ventricular systole: 1. Asynchronous contraction, when separate areas of myocardial wall start to contract and intraventricular pressure rises. 2. Isometric contraction, when the main part of the ventricular myocardium contracts, atrioventricular valves close, and intraventricular pressure significantly increases. 3. The ejection phase, when the intraventricular pressure reaches the pressure in the main vessels, and the semilunar valves open. During diastole (ventricular relaxation): 1. Closure of semilunar valves. 2. Isometric relaxation – initial relaxation of ventricular myocardium, with atrioventricular and semilunar valves closed, until the pressure in the ventricles becomes lower than in the atria. 3. Phases of fast and slow ventricular filling - atrioventricular valves open and blood flows from the atria to the ventricles. 4. Atrial systole, after which cardiac cycle repeats again. The noise produced By a working heart is called heart sounds. In auscultation two sounds can be well heard in healthy subjects: the first sound (S1), which is produced during systole, and the second sound (S2), which occurs during diastole. -

Proceedings of the British Cardiac Society
Br Heart J: first published as 10.1136/hrt.33.1.142 on 1 January 1971. Downloaded from British Heart Journal, 1971, 33, I42-I5 I. Proceedings of the British Cardiac Society THE FORTY-NINTH ANNUAL post. The names receiving the most would be merged with the contingency GENERAL MEETING of the British votes shall be elected in each case at fund. Cardiac Society was held in the Konin- the next Annual General Meeting. In klijk Institut voor de Tropen, Mauri- the event of a draw for any office, the 4 The following resignations were ac- tskade 63, Amsterdam, on Thursday Council shall decide the member to be cepted with regret: Bingham, J. G. M. and Friday, 23 and 24 April I970 as a elected. Hamilton, A. C. Macdonald. joint meeting with the Dutch Society. Rule 25 to be amended as follows: 5 Hamer was elected as Secretary of The President, SIR JOHN Mc- Three Ordinary Members shall be the Society. MICHAEL, took the Chair at 9.oo elected in accordance with Rule I4 a.m. during Private Business before (as amended above) as Secretary, 6 Sowton was elected as Assistant handing over to the Chairman, R. W. D. Assistant Secretary and Treasurer Secretary of the Society. TURNER. respectively. The Secretary shall 7 The following two new Members of hold office for not more than two Council were elected in place of Tubbs years and the Assistant Secretary for Business and A. J. Thomas: Donald Ross and Private not more than two years; the Trea- Byron Evans. i The Minutes of the Annual General surer shall hold office for five years Meeting having been published in the and shall be eligible for re-election. -

Triple Heart Rhythm*
TRIPLE HEART RHYTHM * BY WILLIAM EVANS From the Cardiac Department of The London Hospital Received August 28, 1943 Triple heart rhythm stands for the cadence produced when three sounds recur in successive cardiac cycles, just as two sounds compose the familiar dual rhythm of cardiac auscultation, and more rarely, four sounds a quadruple rhythm. The conflicting views on the subject have long served to discourage attempts at a clinical perception of the problem. Disagreement is perhaps best illustrated by recounting the varied terminology employed to describe it. Thus we have gallop rhythm, canter rhythm, and trot rhythm; presystolic gallop, systolic gallop, protodiastolic gallop, and mesodiastolic gallop; complete summation gallop and incomplete summation gallop; auricular gallop, ventricular gallop, and auriculo-ventricular gallop; true gallop; left-sided gallop and right-sided gallop; rapid-filling gallop; diastolic echo; mitral opening snap; reduplication of first sound and reduplication of second sound; Potain's murmur; third heart sound and fourth heart sound. Others may have escaped my notice. This muddled nomenclature, as long as it stands, will frustrate any attempt to unify the many views held on triple rhythm. There is need of a simplified terminology based on clinical findings. It is indeed clear that a neglect of the clinical aspect on the one hand, and a persistence on the part ofmany to explain the mechanism of the supernumerary sound on the other hand, and to classify triple rhythm in accordance with sound records, have been largely responsible for obscuring this common form of cardiac rhythm. Phonocardiography need not become a routine test in clinical cardiology; when it has helped to establish a classification of triple rhythm it will have achieved its main purpose, though it will still serve in other auscultatory problems. -

AUSCULTATION SKILLS for ATHLETIC TRAINERS
AUSCULTATION SKILLS for ATHLETIC TRAINERS Dennis A. Cardone, DO, CAQSM Director, Sports Medicine and Sports Medicine Fellowship UMDNJ-Robert Wood Johnson Medical School New Brunswick, NJ The Athletes Heart (Athletic Heart Syndrome) I. History a. Dietlen 1908: excessive and continued strain could lead to collapse of the cardiovascular system b. Medical community early 1900s: increased cardiac size in athletes was a pathologic response to the increased cardiac stress of exercise c. Frieberg 1972: cardiovascular changes noted among athletes were more a function of disease (acquired or congenital) than a physiologic response to the adaptations caused by the athlete’s training d. 2004: athletic heart syndrome represents normal physiologic adaptations to training that allows normal or improved cardiac function in contrast to the cardiac dysfunction of pathologic hypertrophy. II. Physiologic response of cardiovascular system to exercise a. Exercise training i. Peripheral response 1. increased number of capillaries, mitochondria, oxidative enzymes 2. improved uptake and utilization of O2 in skeletal muscles ii. Central response (Heart adaptations) 1. increased stroke volume due to cardiac dilation and hypertrophy 2. decrease in resting HR (CO=SV x HR) 3. maximal HRs are same in trained and untrained athletes 4. increased vagal tone III. History: negative IV. Physical examination a. Bradycardia b. Lower BP c. Laterally displaced PMI (because of LVH) d. Auscultation i. Systolic ejection murmur (most intense supine b/c increased left ventricular filling; less intense standing or squatting b/c decreased left ventricular filling) ii. Sinus arrhythmia iii. Wide splitting first heart sound iv. Wide splitting second heart sound v. Filling sounds – S3 ventricular gallop vi. -

Triple Heart Rhythm*
Br Heart J: first published as 10.1136/hrt.5.4.205 on 1 October 1943. Downloaded from TRIPLE HEART RHYTHM * BY WILLIAM EVANS From the Cardiac Department of The London Hospital Received August 28, 1943 Triple heart rhythm stands for the cadence produced when three sounds recur in successive cardiac cycles, just as two sounds compose the familiar dual rhythm of cardiac auscultation, and more rarely, four sounds a quadruple rhythm. The conflicting views on the subject have long served to discourage attempts at a clinical perception of the problem. Disagreement is perhaps best illustrated by recounting the varied terminology employed to describe it. Thus we have gallop rhythm, canter rhythm, and trot rhythm; presystolic gallop, systolic gallop, protodiastolic gallop, and mesodiastolic gallop; complete summation gallop and incomplete summation gallop; auricular gallop, ventricular gallop, and auriculo-ventricular gallop; true gallop; left-sided gallop and right-sided gallop; rapid-filling gallop; diastolic echo; mitral opening snap; reduplication of first sound and reduplication of second sound; Potain's murmur; third heart sound and fourth heart sound. Others may have escaped my notice. This muddled nomenclature, as long as it stands, will frustrate any attempt to unify the many views held on triple rhythm. There is need of a simplified terminology based on clinical findings. It is indeed clear that a neglect of the http://heart.bmj.com/ clinical aspect on the one hand, and a persistence on the part ofmany to explain the mechanism of the supernumerary sound on the other hand, and to classify triple rhythm in accordance with sound records, have been largely responsible for obscuring this common form of cardiac rhythm. -

6 Heart Sounds
Chapter 6 / Heart Sounds 141 6 Heart Sounds CONTENTS PRINCIPLES OF SOUND FORMATION IN THE HEART FIRST HEART SOUND (S1) CLINICAL ASSESSMENT OF S1 AND COMPONENTS SECOND HEART SOUND (S2) NORMAL S2 ABNORMAL S2 CLINICAL ASSESSMENT OF S2 OPENING SNAP (OS) THIRD HEART SOUND (S3) CLINICAL ASSESSMENT OF S3 FOURTH HEART SOUND (S4) CLINICAL ASSESSMENT OF S4 REFERENCES PRINCIPLES OF SOUND FORMATION IN THE HEART In the past, many theories have been advanced to explain the origins of sounds during the cardiac cycle. These included simple concepts of sound originating from the actual contact of valve cusps upon closure. When it was realized that the strength of contraction of the left ventricle had a significant effect on the intensity of the first heart sound, the myocardial theory of the origin of the sound was postulated. Some even had suggested extracardiac origin of sounds such as the third heart sound. It is now, however, well established by several investigators and accepted that the formation of all sounds in the heart can be explained by a “unified concept” (1–10). It is a common experience to hear sound produced when a pipe half-filled with water is moved back and forth, splashing the water against the two palms of the hands held against the ends of the pipe. We have all heard banging sounds sometime produced in the water pipes of the plumbing systems of our homes when air is introduced into the plumb- ing. In both examples, the mechanism of sound production is the same. When the moving column of water in either case comes to sudden stop or marked deceleration, the energy of the column dissipates and in the process generates vibration of the pipes as well as the column of water. -

Cardiac Auscultation: Normal and Abnormal
This is a repository copy of Cardiac auscultation: normal and abnormal. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/145443/ Version: Accepted Version Article: Warriner, D., Michaels, J. and Morris, P.D. orcid.org/0000-0002-3965-121X (2019) Cardiac auscultation: normal and abnormal. British Journal of Hospital Medicine, 80 (2). C28-C31. ISSN 1750-8460 10.12968/hmed.2019.80.2.C28 This document is the Accepted Manuscript version of a Published Work that appeared in final form in British Journal of Hospital Medicine, copyright © MA Healthcare, after peer review and technical editing by the publisher. To access the final edited and published work see https://doi.org/10.12968/hmed.2019.80.2.C28 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Cardiac Auscultation: normal and abnormal Dr David Warriner1, Dr Joshua Michaels2, Dr Paul D Morris3,4 Dr Warriner is a senior cardiology registrar (St7), Dr Michaels is a Foundation Year 2 (FY2) doctor and Dr Morris is NIHR Clinical Lecturer and BCIS Fellow. -
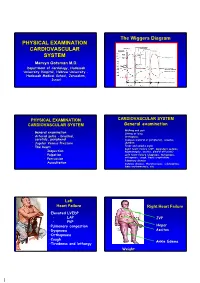
Physical Examination Cardiovascular System Physical Examination Cardiovascular System
The Wiggers Diagram PHYSICAL EXAMINATION CARDIOVASCULAR SYSTEM Mervyn Gotsman M.D. Department of Cardiology, Hadassah x y University Hospital, Hebrew University - Hadassah Medical School, Jerusalem, Israel PHYSICAL EXAMINATION CARDIOVASCULAR SYSTEM CARDIOVASCULAR SYSTEM General examination • Walking and gait • General examination • Sitting or lying • Arterial pulse – brachial, • Orthopnoea carotids, peripheral • Cyanosis (central or peripheral), anaemia, • Jugular Venous Pressure jaundice • Fever and embolic signs • The heart • • • Right heart failure (JVP, dependent oedema, • Inspection hepatomegaly, ascites, pleural effusions) • Palpation • Left heart failure (dyspnoea, tachypnoea, • Percussion orthopnoea, cough, basal crepitations • Pulmonary disease • Auscultation • Sytemic disease: thyrotoxicosis, scleroderma, lupus erythematosis, etc Left Heart Failure Right Heart Failure •Elevated LVEDP • LAP JVP • PVP •Pulmonary congestion Hepar •Dyspnoea Ascites •Orthopnoea •Cough Ankle Edema •Tiredness and lethargy Weight 1 PHYSICAL EXAMINATION AArterialrterial pulse CARDIOVASCULAR SYSTEM Radial artery • Arterial pulse • Jugular Venous Pressure • Cardiac Examination: • Inspection • Palpation • Percussion • Auscultation Arterial pulse 120 Arterial pulse Radial artery 80 Carotid artery Amplitude/Contour: • Heart rate: ( 60-100 ). • Hypokinetic ↓ ( weak ) - Hypovolemia • Rhythm: Regular 120 Irregular: Sinus arrhythmia Heart failure Occasional - Premature beats 80 Aortic stenosis - Dropped beats • Hyperkinetic ↑ increased stroke volume Totally - -

SAM II, on Line Menu of Sounds Heart Sounds Group Description Heart
SAM II, On Line Menu of Sounds Heart Sounds Group Description Heart Sounds, Normal & Variants Normal Heart Sounds, 60 bpm Normal Heart Sounds, 75 bpm Normal Heart Sounds, 90 bpm Normal Heart Sounds, 110 bpm Third Heart Sound, Pediatric, 90 bpm Fourth Heart Sound, 80 bpm Physiological Splitting of Second Sound Paradoxical Splitting of Second Sound Aortic Heart Sounds Aortic Stenosis, Mild Aortic Stenosis, Severe, S2 Absent Ex1 Aortic Stenosis, Severe Ex2 Aortic Stenosis and Regurgitation Congenital Aortic Stenosis Acute Aortic Regurgitation Mitral Heart Sounds Mitral Valve Prolapse Mitral Regurgitation, Mild Mitral Regurgitation, Marked Mitral Stenosis Mitral Stenosis and Regurgitation Pediatric Heart Sounds Innocent Systolic Murmur Innocent Systolic Murmur - Vibratory Ventricular Septal Defect Atrial Septal Defect Patent Ductus Arteriosus Pulmonary Hypertension Tetralogy of Fallot Summation Sound Eisenmenger's Syndrome Other Heart Sound Pulmonary Stenosis with Intact Septum Acute Pericarditis Hyptertrophic Cardiomyopathy Congestive Heart Failure Atrial Fibrillation Venous Hum Coarctation of the Aorta BRUITS BRUITS Carotid Bruit, Mild I Carotid Bruit, Medium I Carotid Bruit, Mild II Carotid Bruit, Medium II Aortic Aneurysm Renal Bruit HEART-LUNG COMBINATIONS Heart-Lung Combinations Aortic Stenosis with normal breath sounds Aortic Stenosis with Crackles Atrial Fibrillation Fourth Heart Sound with respiatory variation Mitral Regurg with normal breath sounds Mitral Regurg with crackles Mitral Valve Proplapse with breath sounds Normal Heart