Investigation of Soil Characters and Azospirillum Isolated from Paddy Soils of Thanjavur District,East Coast of Tamilnadu, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Telephone Numbers
DISTRICT DISASTER MANAGEMENT AUTHORITY THANJAVUR IMPORTANT TELEPHONE NUMBERS DISTRICT EMERGENCY OPERATION CENTRE THANJAVUR DISTRICT YEAR-2018 2 INDEX S. No. Department Page No. 1 State Disaster Management Department, Chennai 1 2. Emergency Toll free Telephone Numbers 1 3. Indian Meteorological Research Centre 2 4. National Disaster Rescue Team, Arakonam 2 5. Aavin 2 6. Telephone Operator, District Collectorate 2 7. Office,ThanjavurRevenue Department 3 8. PWD ( Buildings and Maintenance) 5 9. Cooperative Department 5 10. Treasury Department 7 11. Police Department 10 12. Fire & Rescue Department 13 13. District Rural Development 14 14. Panchayat 17 15. Town Panchayat 18 16. Public Works Department 19 17. Highways Department 25 18. Agriculture Department 26 19. Animal Husbandry Department 28 20. Tamilnadu Civil Supplies Corporation 29 21. Education Department 29 22. Health and Medical Department 31 23. TNSTC 33 24. TNEB 34 25. Fisheries 35 26. Forest Department 38 27. TWAD 38 28. Horticulture 39 29. Statisticts 40 30. NGO’s 40 31. First Responders for Vulnerable Areas 44 1 Telephone Number Officer’s Details Office Telephone & Mobile District Disaster Management Agency - Thanjavur Flood Control Room 1077 04362- 230121 State Disaster Management Agency – Chennai - 5 Additional Cheif Secretary & Commissioner 044-28523299 9445000444 of Revenue Administration, Chennai -5 044-28414513, Disaster Management, Chennai 044-1070 Control Room 044-28414512 Emergency Toll Free Numbers Disaster Rescue, 1077 District Collector Office, Thanjavur Child Line 1098 Police 100 Fire & Rescue Department 101 Medical Helpline 104 Ambulance 108 Women’s Helpline 1091 National Highways Emergency Help 1033 Old Age People Helpline 1253 Coastal Security 1718 Blood Bank 1910 Eye Donation 1919 Railway Helpline 1512 AIDS Helpline 1097 2 Meteorological Research Centre S. -

ANSWERED ON:11.05.2005 AUTOMATIC and MODERN TELEPHONE EXCHANGES in TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy
GOVERNMENT OF INDIA COMMUNICATIONS AND INFORMATION TECHNOLOGY LOK SABHA UNSTARRED QUESTION NO:6879 ANSWERED ON:11.05.2005 AUTOMATIC AND MODERN TELEPHONE EXCHANGES IN TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy Will the Minister of COMMUNICATIONS AND INFORMATION TECHNOLOGY be pleased to state: (a) the details of automatic and modern telephone exchanges set up in Tamil Nadu during the last three years, location- wise; (b) the details of such exchanges proposed to be set up in Tamil Nadu during the current year; (c) the details of the telephone exchanges whose capacities were expanded in the current financial year; and (d) the details of telephone exchanges where waiting list for telephone connection still exists? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS ANDINFORMATION TECHNOLOGY (DR. SHAKEEL AHMAD) (a) The details of automatic and modern telephone exchanges set up in Tamilnadu during the last three years are given in the Annexures- I(a), I(b) & I(c). (b) The details of such exchanges proposed to be set up in Tamilnadu during the current year are given in Annexure-II. (c) The details of the telephone exchanges whose capacities were expanded in the current financial year are given at Annexure-III. (d) The details of telephone exchanges where waiting list for telephone connection still exists are given in Annexure- IV. ANNEXURE-I(a) DETAILS OF TELEPHONE EXCHANGES SET UP DURING 2002-03 IN TAMILNADU Sl Name of Exchange Capacity Type/Technology District No.(Location) 1 Avinashi-II 4000 CDOTMBMXL Coimbatore 2 K.P.Pudur -

Chapter - Iv Profile of the Study Area
CHAPTER - IV PROFILE OF THE STUDY AREA THANJAVUR DISTRICT PROFILE The study area chosen for the research is Thanjavur District, Tamil Nadu. Thanjavur district is bounded on the north east by Nagapattinam district, north be Tiruchirappalli, Perabalur and Cuddalore districts, east by Thiruvarur district, south-east by Palk strait, west by Pudukkottai and north-west by Tiruchirappalli district. Geocode North Latitude 10o 08’ to 11o 12’ East longitude 78o 48’ to 79o 38’ Agro Ecological Region : Region 8 : Semi-arid ecosystem (90%) – Eastern ghats, Tamil Nadu uplands – hot semiarid ecosystem with a crop growing period of 90 to 120 days. Region 18 : Coastal ecosystem (10%) – Eastern coastal plain hot sub – humid to semi-arid ecoregion and with a crop growing period of 90 to 210 days. Agroclimatic zone : Sub zone IV – Cauvery delta zone. 77 Table 4.1 Taluks and Panchayat Unions S.No Taluks Panchayat Unions 1. Thanjavur Thanjavur & Budalur (Part) 2. Thiruvaiyaru Thiruvaiyaru & Budalur (Part) 3. Orathanadu Orathanadu & Thiruvonam 4. Kumbakonam Kumbakonam & Thiruvidaimarudhur (Part) 5. Thiruvidaimarudhur Thiruvidaimarudhur (Part) & Thirupanandal 6. Papanasam Papanasam & Ammapettai 7. Pattukkottai Pattukkottai, Peravurani (part) Madukkur 8. Peravurani Peravurani (part) & Sethubavachatram Roads and Railways Thanjavur districts is well connected with a net work of roads and railways. The total length of the road in the district is 2014 km with concrete, black topped, metal and non metaled roads. The important roads are as follows. State highways Tiruchirapalli to Nagapattinam road via Thanjavur Thanjavur to Thiruvaiyaru and Thanjavur to Pudukkottai. The major district roads connect Thanjavur with all taluk headquarters. 78 The district is served by both metre and broad gauge railways (Southern Railways) to a total length of 151km having 27 railways stations with one junction viz., Thanjavur. -

Guide to 275 SIVA STHALAMS Glorified by Thevaram Hymns (Pathigams) of Nayanmars
Guide to 275 SIVA STHALAMS Glorified by Thevaram Hymns (Pathigams) of Nayanmars -****- by Tamarapu Sampath Kumaran About the Author: Mr T Sampath Kumaran is a freelance writer. He regularly contributes articles on Management, Business, Ancient Temples and Temple Architecture to many leading Dailies and Magazines. His articles for the young is very popular in “The Young World section” of THE HINDU. He was associated in the production of two Documentary films on Nava Tirupathi Temples, and Tirukkurungudi Temple in Tamilnadu. His book on “The Path of Ramanuja”, and “The Guide to 108 Divya Desams” in book form on the CD, has been well received in the religious circle. Preface: Tirth Yatras or pilgrimages have been an integral part of Hinduism. Pilgrimages are considered quite important by the ritualistic followers of Sanathana dharma. There are a few centers of sacredness, which are held at high esteem by the ardent devotees who dream to travel and worship God in these holy places. All these holy sites have some mythological significance attached to them. When people go to a temple, they say they go for Darsan – of the image of the presiding deity. The pinnacle act of Hindu worship is to stand in the presence of the deity and to look upon the image so as to see and be seen by the deity and to gain the blessings. There are thousands of Siva sthalams- pilgrimage sites - renowned for their divine images. And it is for the Darsan of these divine images as well the pilgrimage places themselves - which are believed to be the natural places where Gods have dwelled - the pilgrimage is made. -
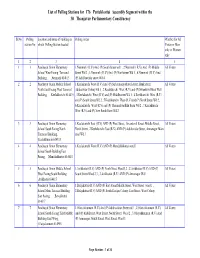
List of Polling Stations for 176 Pattukkottai Assembly Segment Within the 30 Thanjavur Parliamentary Constituency
List of Polling Stations for 176 Pattukkottai Assembly Segment within the 30 Thanjavur Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 12 3 4 5 1 1 Panchayat Union Elementary 1.Nemmeli ( R.V) And (P) South Street wd 1 , 2.Nemmeli ( R.V) And (P) Middle All Voters School West Facing Terraced Street Wd 2 , 3.Nemmeli ( R.V) And (P) Northstreet Wd 3 , 4.Nemmeli ( R.V) And Building, ,Nemmeli 614015 (P) Adi Dravidar street Wd 4 2 2 Panchayat Union Middle School 1.Keelakurichi West (R.V) And (P) Subramaniyarkovil street, Bank street, All Voters North East Facing West Terraced Adidravidar Colony wd 1 , 2.Keelakurichi West (R.V) and (P) Sivankovil Steet Wd 1 Building, ,Keelakkurichi 614015 , 3.Keelakurichi West (R.V) and (P) Middlestreet Wd 1 , 4.Keelakurichi West (R.V) and (P) South Street Wd 2 , 5.Keelakurichi West (R.V) and (P) North Street Wd 2 , 6.Keelakurichi West (R.V) and (P) Thenmelavadkku theru Wd 2 , 7.Keelakurichi West (R.V) and (P) New South Steet Wd 2 3 3 Panchayat Union Elementay 1.Keelakurichi East (R.V) AND (P) West Street, Sivankovil Street, Middle Street, All Voters School South Facing North North Street , 2.Keelakurichi East (R.V) AND (P) Adidravidar Street, Annanagar Main Terrraced Building, road Wd 3 ,Keelakkurichi 614015 4 4 Panchayat Union Elementary 1.Keelakurichi West (R.V) AND (P) Mandalakkottai ward 1 All Voters School South Building East Facing, ,Mandalakkottai 614015 5 5 Panchayat Union -

Surface and Sub Surface Soil Mapping Using Geological and Geotechnical Investigation for Western Cauvery Delta, Thanjavur and Thiruvarur Districts, Tamil Nadu, India
IOSR Journal of Applied Geology and Geophysics (IOSR-JAGG) e-ISSN: 2321–0990, p-ISSN: 2321–0982.Volume 4, Issue 1 Ver. I (Jan. - Feb. 2016), PP 49-57 www.iosrjournals.org Surface and sub surface soil mapping using geological and geotechnical investigation for Western Cauvery Delta, Thanjavur and Thiruvarur Districts, Tamil Nadu, India. Mr C. Sankar.¹ Dr S. Senthamil Kumar.² Dr. C. Lakshumanan³ ¹Department of Civil engineering, Periyar Maniammai University. Vallam. Thanjavur. 613 403. ²Centre for Climate change, Periyar Maniammai University. Vallam. Thanjavur. 613 403. ³Centre for Remote Sensing, Bharathidasan University, Tiruchirappalli – 620023. Abstract: Subsurface investigation is ascertaining, Properties and types of soil at various depths in study area. This finding is useful for geologist, geotechnical engineers and Agricultural scientist. Selected site in study area, the envoy sub surface soil sample has collected with help of Standard Penetration Test (SPT) sampling techniques. Collected sub surface soil sample has tested in Laboratory and resulting soil properties are tabulated. From geo referenced Tamil Nadu soil prepared by National Bureau of soil survey and Land use Planning (ICAR), Nagpur, Department of Agriculture, Tamil Nadu, the soil unit has mapped with help of Arc MAP. Study area Key map, soil sample location map has prepared From LISS IV image data and Survey of India Topo sheets. Using Garmin GPS the Geo coordinate has observed while sampling on different site on the Study area. Key Words: Arc map, Density, Geo coordinate, GPS, Sampling and SPT. I. Introduction Soil is most important after Air and Water in the earth, more than 90% world food production is dependent on soil. -

Dynamics of Landuse and Landcover Changes in Papanasam Taluk, Thanjavur District, Tamilnadu, India
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2016, 7(2):128-131 ISSN: 0976-8610 CODEN (USA): AASRFC Dynamics of landuse and landcover changes in Papanasam Taluk, Thanjavur District, Tamilnadu, India G. Laxmi, K. Indhira, J. Senthil * and P. H. Anand Department of Geography, Govt. Arts College (A), Kumbakonam, Tamil Nadu, India _____________________________________________________________________________________________ ABSTRACT Remote sensing has been in common use to provide the primary data from which land use and land cover types and their boundaries are for interpretation purpose. During the past four decades several surveys, studies, and other projects have successfully demonstrated that remote sensing data are useful for land use and land cover inventory and mapping. These surveys have contributed to our confidence that land use and land cover surveys of larger areas are possible by the use of remote sensing data bases. It has been argued in this paper that a systematic analysis of local-scale land-use change studies, conducted over a range of timescale. Over the last few decades, numerous researchers have improved measurements of land-cover change, the understanding of the causes of land-use change. Key words: Landuse, Landcover, Remote sensing _____________________________________________________________________________________________ INTRODUCTION Present use of land is one of the characteristics that are widely recognized as significant for planning and management purposes. One concept that has much merit is that land use refers to, "man's activities on land which are directly related to the land" [1]. Land cover, on the other hand, describes, "the vegetation and artificial constructions covering the land surface" [2]. -

Trichy FOC Centres Phone Numbers Land Line Mobile 9445853
Name of the Region : Trichy Fuse Off Call Centres Name of the Circle : Trichy Phone Numbers FOC Centres Land Line Mobile For BSNL Users:1912 9445853479 - TRICHY For others:04311912 9445853480 Name of the Circle : Karur Phone Numbers FOC Centres Land Line Mobile KARUR 1912 94445854093 Name of the Circle : Pudukkottai Phone Numbers FOC Centres Land Line Mobile Pudukkottai 04322-221523 ----- Landline Numbers Name of the Circle : TRICHY Elecy. Distn. Circle/Metro Section Sub-Division Division Section Name Phone No Sub Division Phone No Division Phone No Name Name Thennur 0431-2794237 Thillainagar 0431-2791467 0431 - Woraiyur 0431 -2794237 THENNUR 2794237 Srinivasanagar 0431 -2794237 Con-II/Rockfort 0431-2793220 Con-I/Urban/Trichy 0431-2793220 Rockfort 0431-2793220 0431- Cinthamani 0431 -2793220 ROCKFORT 0431 - 2793220 Maingauardgate 0431 -2793220 URBAN 2793131 110KV/K.Pettai 0431 -2706443 Palakkarai 0431-2793220 Gandhimarket 0431-2793220 Senthaneerpuram 0431 -2793220 0431 - PALAKKARAI Junction 0431 -2414749 2793220 Ponnagar 0431-2481858 Mahalakshminagar 0431 -2202525 Cantonment 0431-2460148 Mannarpuram 0431-2420145 Subramaniapuram 0431 -2420145 Up graded Code No: Sembattu 0431 -2341924 section 0431 Crawford 0431 -2471880 KK Nagar 0431 -2341032 Rural/ Trichy 0431-2422301 EAST 0431 Manikandam 0431-2680300 /TRICHY 242223 Tiruparaithurai 0431-2614322 RURAL / 0431- TRICHY 2422301 Somarasampettai 0431-2607271 110 KV SS/ Ammapettai 0431-2680300 110 KV SS/Alundur 0431-2680514 Tiruverumbur 0431-2512773 THIRUVERUMB 0431- Navalpattu 0431-2512224 UR -

A Perspective on Pilgrimage Tourism in Thanjavur District
International Journal of Applied Research 2016; 2(4): 116-120 ISSN Print: 2394-7500 ISSN Online: 2394-5869 A perspective on pilgrimage tourism in Thanjavur Impact Factor: 5.2 IJAR 2016; 2(4): 116-120 district www.allresearchjournal.com Received: 12-02-2016 Accepted: 15-03-2016 Dr. Balu A, Senthilkumar A Dr. Balu A Assistant Professor & Research Abstract Advisor PG & Research In the rapidly changing global economic scenario, tourism is considered to be one of the largest and fast Department of Commerce growing industries. Thanjavur is the headquarters of Thanjavur district of Tamil Nadu state. There are Government Arts College (A) numerous ancient temples in the district and also the famous Mahamaham Tank in Kumbakonam. Kumbakonam – 612002. Tourism provides very useful and fruitful avenues especially to those people who are engaged in tourism activities because through this smokeless industry, they are not only enhancing their standard A Senthilkumar of living but also generating income and employment opportunities. In the contemporary era of Full time Research Scholar globalization and industrialization, the whole world has shrunk into a global village. Tourism has PG & Research Department of Commerce Government Arts played a very decisive role to transform the world into a globalized economy where all nations can College (A) Kumbakonam – exchange free trade and culture and share their interest of mutual benefits based on tourism industry. 612002. India is one of the major destinations of the foreign tourists particularly as it is a country that has a rich cultural heritage. Tourists visit Thanjavur for its scenic beauty, navagraha temples and ecological resources. -

Thanjavur District
Village Panchayat Details Sl.No Name of the Block Name of the Village Panchayat 1 Ammapettai AGARAMANGUDI 2 ALANGUDI 3 ANNAPPANPETTAI 4 ARUMALAIKKOTTAI 5 ARUNDAVAPURAM 6 DEVARAYANPETTAI 7 EDAIYIRUPPU 8 EDAVAKKUDI 9 IRUMBUTHALAI 10 JANBAGAPURAM 11 KALANCHERI 12 KAVALUR 13 KAMBARNATHAM 14 KARUPPAMUDALIYARKOTTAI 15 KATHIRINATHAM 16 KEELAKOILPATHU 17 KOTHANGUDI 18 KUMILAKUDI 19 MAKIMALAI 20 MELAKALAKUDI 21 MELASEMMANGUDI 22 KOVATHAGUDI 23 NALLAVANNIANKUDIKADU 24 NEDUVASAL 25 NEIKKUNNAM 26 NELLITHOPPU 27 OMPATHUVELI 28 PALLIYUR 29 PERUMAKKANALLUR 30 POONDI 31 PULAVARNATHAM 32 PULIYAKKUDI 33 RARAMUTHIRAKKOTTAI 34 SALIAMANGALAM 35 SERUMAKKANALLUR 36 SULIAKKOTTAI 37 SURAIKKAYUR 38 THIRUBUVANAM 39 THIRUKKARUGAVUR 40 THIRUVAIYATHUKUDI 41 UKKADAI 42 VADAKKU MANGUDI 43 VADAPATHI 44 VAIYACHERI 45 VEMBUGUDI 46 VILUTHIYUR 47 Budalur ACHAMPATTI 48 AGARAPETTAI 49 ALAMELUPURAM 50 ARCADU 51 AVARAMPATTI 52 BUDALUR 53 DEEKSHASAMUDRAM 54 INDALUR 55 KADAMBANGUDI 56 KANKEYANPATTI 57 KATCHAMANGALAM 58 KOOTHUR 59 KOVILADI 60 KOVILPATHU 61 MAICKELPATTI 62 MANIYERIPATTI 63 MARANERI 64 MEGALATHUR 65 MUTHUVEERAKANDIANPATTI 66 NANDAVANAPATTI 67 NEMAM 68 ORATHUR 69 PALAMANERI 70 PALAYAPATTI (NORTH) 71 PALAYAPATTI (SOUTH) 72 PATHIRAKKUDI 73 PAVANAMANGALAM 74 PUDUKKUDI 75 PUDUPATTI 76 RAJAGIRI 77 RANGANATHAPURAM 78 SANOORAPATTI 79 SELLAPPANPETTAI 80 SENGIPATTI 81 SOLAGAMPATTI 82 THIRUCHINAMPOONDI 83 THOGUR 84 THONDARAYANPADI 85 VEERAMARASANPETTAI 86 VENDAYAMPATTI 87 VISHNAMPETTAI 88 VITTALAPURAM 89 Kumbakonam AMMACHATRAM 90 ANNALAGRAHARAM 91 ARIYAPADAIVEEDU 92 ASOOR 93 -

Papanasam Assembly Tamil Nadu Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781, 26810964-65-66 Email : [email protected] Website : www.electionsinindia.com Online Book Store : www.datanetindia-ebooks.com Report No. : AFB/TN-172-0619 ISBN : 978-93-5313-821-9 First Edition : January, 2018 Third Updated Edition : June, 2019 Price : Rs. 11500/- US$ 310 © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 182 for the use of this publication. Printed in India No. Particulars Page No. Introduction 1 Assembly Constituency - (Vidhan Sabha) at a Glance | Features of Assembly 1-2 as per Delimitation Commission of India (2008) Location and Political Maps Location Map | Boundaries of Assembly Constituency - (Vidhan Sabha) in 2 District | Boundaries of Assembly Constituency under Parliamentary 3-9 Constituency - (Lok Sabha) | Town & Village-wise Winner Parties- 2019, 2016, 2014, 2011 and 2009 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 10-16 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population | Towns and -

Economic and Cultural History of Tamilnadu from Sangam Age to 1800 C.E
I - M.A. HISTORY Code No. 18KP1HO3 SOCIO – ECONOMIC AND CULTURAL HISTORY OF TAMILNADU FROM SANGAM AGE TO 1800 C.E. UNIT – I Sources The Literay Sources Sangam Period The consisted, of Tolkappiyam a Tamil grammar work, eight Anthologies (Ettutogai), the ten poems (Padinen kell kanakku ) the twin epics, Silappadikaram and Manimekalai and other poems. The sangam works dealt with the aharm and puram life of the people. To collect various information regarding politics, society, religion and economy of the sangam period, these works are useful. The sangam works were secular in character. Kallabhra period The religious works such as Tamil Navalar Charital,Periyapuranam and Yapperumkalam were religious oriented, they served little purpose. Pallava Period Devaram, written by Apper, simdarar and Sambandar gave references tot eh socio economic and the religious activities of the Pallava age. The religious oriented Nalayira Tivya Prabandam also provided materials to know the relation of the Pallavas with the contemporary rulers of South India. The Nandikkalambakam of Nandivarman III and Bharatavenba of Perumdevanar give a clear account of the political activities of Nandivarman III. The early pandya period Limited Tamil sources are available for the study of the early Pandyas. The Pandikkovai, the Periyapuranam, the Divya Suri Carita and the Guruparamparai throw light on the study of the Pandyas. The Chola Period The chola empire under Vijayalaya and his successors witnessed one of the progressive periods of literary and religious revival in south India The works of South Indian Vishnavism arranged by Nambi Andar Nambi provide amble information about the domination of Hindu religion in south India.