Measurement of Odor Threshold by Triangle Odor Bag Method
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

N I W O 2011/152886
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date / n / i 8 December 2011 (08.12.2011) W O 2011/152886 A 2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61L 9/01 (2006.01) A61K 31/23 (2006.01) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, A61K 31/221 (2006.01) A61Q 15/00 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 31/22 (2006.01) A61Q 13/00 (2006.01) HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (21) International Application Number: ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, PCT/US201 1/022697 NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (22) International Filing Date: SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 27 January 201 1 (27.01 .201 1) TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, (30) Priority Data: ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, 61/35 1,435 4 June 2010 (04.06.2010) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, ΓΓ 12/974,7 16 2 1 December 2010 (21 .12.2010) US EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, , LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, (71) Applicant (for all designated States except US): SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, ROBERTET, INC. -

Medical Review Officer Manual
Department of Health and Human Services Substance Abuse and Mental Health Services Administration Center for Substance Abuse Prevention Medical Review Officer Manual for Federal Agency Workplace Drug Testing Programs EFFECTIVE OCTOBER 1, 2010 Note: This manual applies to Federal agency drug testing programs that come under Executive Order 12564 dated September 15, 1986, section 503 of Public Law 100-71, 5 U.S.C. section 7301 note dated July 11, 1987, and the Department of Health and Human Services Mandatory Guidelines for Federal Workplace Drug Testing Programs (73 FR 71858) dated November 25, 2008 (effective October 1, 2010). This manual does not apply to specimens submitted for testing under U.S. Department of Transportation (DOT) Procedures for Transportation Workplace Drug and Alcohol Testing Programs (49 CFR Part 40). The current version of this manual and other information including MRO Case Studies are available on the Drug Testing page under Medical Review Officer (MRO) Resources on the SAMHSA website: http://www.workplace.samhsa.gov Previous Versions of this Manual are Obsolete 3 Table of Contents Chapter 1. The Medical Review Officer (MRO)........................................................................... 6 Chapter 2. The Federal Drug Testing Custody and Control Form ................................................ 7 Chapter 3. Urine Drug Testing ...................................................................................................... 9 A. Federal Workplace Drug Testing Overview.................................................................. -

Evaluation of Ozonation on Levels of the Off-Flavor Compounds Geosmin and 2-Methylisoborneol in Water and Rainbow Trout Oncorhyn
Aquacultural Engineering 43 (2010) 46–50 Contents lists available at ScienceDirect Aquacultural Engineering journal homepage: www.elsevier.com/locate/aqua-online Evaluation of ozonation on levels of the off-flavor compounds geosmin and 2-methylisoborneol in water and rainbow trout Oncorhynchus mykiss from recirculating aquaculture systems Kevin K. Schrader a,∗, John W. Davidson b, Agnes M. Rimando a, Steven T. Summerfelt b a United States Department of Agriculture, Agricultural Research Service, Natural Products Utilization Research Unit, National Center for Natural Products Research, Post Office Box 8048, University, MS 38677-8048, USA View metadata, citation and similar papers at core.ac.uk brought to you by CORE b The Conservation Fund Freshwater Institute, 1098 Turner Road, Shepherdstown, WV 25443, USA provided by Elsevier - Publisher Connector article info abstract Article history: Common “off-flavors” in fish cultured in recirculating aquaculture systems (RAS) are “earthy” and “musty” Received 29 January 2010 due to the presence of the off-flavor metabolites geosmin and 2-methylisoborneol (MIB), respectively. Accepted 17 May 2010 Previously, ozone addition has been applied to RAS at relatively low doses to break refractory organic molecules (i.e., reducing color), microflocculate fine particulate matter (i.e., increasing solids removal), Keywords: and oxidize nitrite to nitrate, but the effect of ozone addition at these dosing rates on levels of off-flavor Aquaculture compounds was unknown. Ozonation has been used in municipal drinking water facilities to reduce the Geosmin levels of these compounds, to improve water taste, and to subsequently reduce consumer complaints. In 2-Methylisoborneol Off-flavor this study, the effects of ozone addition to the inlet water of the RAS culture tanks on levels of geosmin Ozone and MIB in the culture water and fish flesh were evaluated. -

Geosmin and 2-MIB Removal by Full-Scale Drinking Water Treatment Processes in the Republic of Korea
water Article Geosmin and 2-MIB Removal by Full-Scale Drinking Water Treatment Processes in the Republic of Korea Keug Tae Kim 1 and Yong-Gyun Park 2,* 1 Department of Environmental & Energy Engineering, Suwon University, 17 Wauan-gil, Bongdam-eup, Hwaseong-si 18323, Korea; [email protected] 2 Pioneer Team Research Institute, GS Engineering &Construction, 33, Jong-ro, Jongro-gu, Seoul 03159, Korea * Correspondence: [email protected] Abstract: Due to climate change, population growth, industrialization, urbanization, and water contamination, it is becoming more difficult to secure and supply clean and safe drinking water. One of the challenges many water utilities often face is the taste and odor (T&O) problem in drinking water treatment plants, mostly associated with geosmin and 2-MIB. These representative T&O compounds are mainly produced by the metabolism of blue-green algae (cyanobacteria), especially in summer. In this study, the correlation between algae blooms and T&O compounds was identified in the intake and raw water of a large-scale water treatment plant in the Republic of Korea. The removal efficiency of geosmin and 2-MIB by each treatment process was intensively evaluated. According to the obtained results, ozonation and granular activated carbon (GAC) adsorption were more effective for removing the troublesome compounds compared to other water treatment processes, such as coagulation/flocculation, filtration, and chlorination. Because of their seasonal concentration variation and different removal rates, optimal operation methods need to be developed and implemented for drinking water treatment plants to solve the T&O problems. Citation: Kim, K.T.; Park, Y.-G. -
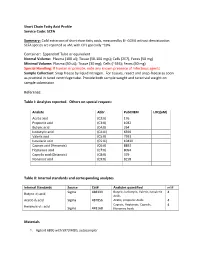
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -
![[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An](https://docslib.b-cdn.net/cover/9902/agr-biol-chem-vol-36-no-1-p-112-119-1972-studies-of-the-peptide-antibiotic-suzukacillin-part-ii-by-takaaki-ooka-an-399902.webp)
[Agr. Biol. Chem., Vol. 36, No. 1, P. 112---119, 1972] Studies of the Peptide Antibiotic Suzukacillin Part II by Takaaki OOKA An
[Agr. Biol. Chem., Vol. 36, No. 1, p. 112---119,1972] Studies of the Peptide Antibiotic Suzukacillin Part II By Takaaki OOKA and Isao TAKEDA TechnicalResearch Laboratory, Asahi ChemicalIndustry Co., Ltd., Nakadai-cho3-27, Itabashi-kuTokyo ReceivedJuly 26, 1971 Suzukacillin produced by Trichoderma viride 6301 strain was purified and shown to be separated into two components A and B on thin-layer chromatography. The component A was isolated and crystallized from the mixture of components by alumina column chro matography. The component A is composed of six amino acids, Gly, Glu, Ala, Pro, Val, Leu and an unknown amino acid. This unknown amino acid was identified as a-amino isobutyric acid. It is supposed that a-amino isobutyrie acid is biosynthesized mainly from L-valine by the isotopic experiments. Suzukacillin formation by Trichoderma viride 6301 was stimulated by the addition of L-Asn, GABA, L-Ser, Gly and L-Arg into the medium. Trichoderma viride 6301 strain producing Suzu ments were purchased as reagents from Tokyo Kasei kacillin was isolated by the author's laboratory. Co., Ltd. The following radioactive compounds were This organism accumulated noted amount of surplied by Dai-ichi Chemical Co., Ltd., with the in the peptide antibiotic. It was detected on dicated specific activities (ƒÊCi per mmole): Starch- TLC that the peptide antibiotic Suzukacillin (U)14C (30.8), pyruvic acid-(U)-14C (31.5), glycine- consists of two components A and B. A is (U)14C (45.2), L-alanine-(U)-14C (107), L-glutamic acid- (U)-14C (165), L-proline-(U)-14C (165), L-valine-(U)-14C a major and B is a minor component. -

Color, Taste, and Odor: What You Should Know
Color, Taste, and Odor: What you should know From time to time the MassDEP receives consumer questions or complaints regarding the look, taste or the odor of drinking water. Listed below are common problems with drinking water and their most common causes. Please note that a particular problem in your drinking water may be the result of a cause not listed here; the only way to confirm a cause is to have a certified lab analyze the water and discuss the results with drinking water professional. If you receive water from a public drinking water system it is important to contact the Public Water Supply (PWS) before having a laboratory analyze the water. Information on private water testing is available. Filtering or treating the water may remedy persistent problems; however MassDEP does not recommend filtering or treating your water supply if your water is supplied by a MassDEP- approved PWS. MassDEP also does not regulate or recommend specific treatment systems for private home use. If you decide to use a filtration or treatment device in your home, the Department strongly encourages you to contact National Sanitation Foundation (NSF) for a list of approved devices. If you purchase a treatment device for private home use MassDEP also strongly recommends that it is maintained and provide active maintenance according to the manufacturer's instructions. Failure to maintain the equipment properly may make treatment ineffective and/or may create the potential for contamination. Common problems with drinking water are grouped into three categories: Color problems Taste / odor problems Particles in water If the problem with your water is not described here, if you are on a public water system please contact the public water department in your city or town or the MassDEP Drinking Water Program at your nearest regional MassDEP office. -

Water Quality and Relation to Taste-And-Odor Compounds in the North Fork Ninnescah River and Cheney Reservoir South-Central Kans
Prepared in cooperation with the City of Wichita, Kansas Water Quality and Relation to Taste-and-Odor Compounds in the North Fork Ninnescah River and Cheney Reservoir, South-Central Kansas, 1997–2003 Scientific Investigations Report 2006−5095 U.S. Department of the Interior U.S. Geological Survey Water Quality and Relation to Taste-and- Odor Compounds in the North Fork Ninnescah River and Cheney Reservoir, South-Central Kansas, 1997–2003 By Victoria G. Christensen, Jennifer L. Graham, Chad R. Milligan, Larry M. Pope, and Andrew C. Ziegler Prepared in cooperation with the City of Wichita, Kansas Scientific Investigations Report 2006–5095 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior P. Lynn Scarlett, Acting Secretary U.S. Geological Survey P. Patrick Leahy, Acting Director U.S. Geological Survey, Reston, Virginia: 2006 For sale by U.S. Geological Survey, Information Services Box 25286, Denver Federal Center Denver, CO 80225 For more information about the USGS and its products: Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov/ Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Christensen, V.G., Graham, J.L., Milligan, C.R., Pope, L.M., and Ziegler, A.C., 2006, Water quality and relation to taste- and-odor compounds in the North Fork Ninnescah River and Cheney Reservoir, south-central Kansas, 1997–2003: U.S. -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 16-Feb-2021 Version 7.01 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name (±)-Geosmin-d3 Standard Solution (1mg/mL Methanol Solution) Product code 072-06081 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research use only restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Flammable liquids Category 2 Acute toxicity - Oral Category 4 Serious eye damage/eye irritation Category 2A Reproductive Toxicity Category 1B Specific target organ toxicity (single exposure) Category 1, Category 3 Category 1 central nervous system, Visual organ, systemic toxicity Category 3 Narcotic effects Specific target organ toxicity (repeated exposure) Category 1 Category 1 central nervous system, Visual organ Pictograms Signal word Danger Hazard statements H225 - Highly flammable liquid and vapor H319 - Causes serious eye irritation H302 - Harmful if swallowed H360 - May damage fertility or the unborn child H336 - May cause drowsiness or dizziness H370 - Causes damage to the following organs: central nervous system, Visual organ, systemic toxicity H372 - Causes damage to the following organs through -

STD Glossary of Terms
STD 101 In A Box- STD Glossary of Terms Abstinence Not having sexual intercourse Acquired A disease of the human immune system caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS represents the entire range of Immunodeficiency disease caused by the HIV virus from early infection to late stage Syndrome (AIDS) symptoms. Anal Intercourse Sexual contact in which the penis enters the anus. Antibiotic A medication that either kills or inhibits the growth of a bacteria. Antiviral A medication that either kills or inhibits the growth of a virus. A thinning of tissue modified by the location. In epidermal atrophy, the epidermis becomes transparent with a loss of skin texture and cigarette Atrophic paper-like wrinkling. In dermal atrophy, there is a loss of connective tissue and the lesion is depressed. A polymicrobial clinical syndrome resulting from replacement of the Bacterial Vaginosis normal hydrogen peroxide producing Lactobacillus sp. in the vagina with (BV) high concentrations of anaerobic bacteria. The common symptom of BV is abnormal homogeneous, off-white, fishy smelling vaginal discharge. Cervical Motion A sign found on pelvic examination suggestive of pelvic pathology; when Tenderness (CMT) movement of the cervix during the bimanual exam elicits pain. The lower, cylindrical end of the uterus that forms a narrow canal Cervix connecting the upper (uterus) and lower (vagina) parts of a woman's reproductive tract. The most common sexually transmitted bacterial infection in the U.S., caused by the bacteria Chlamydia trachomatis. Often no symptoms are present, especially in women. Untreated chlamydia can cause sterility, Chlamydia Pelvic Inflammatory Disease (PID), and increase the chances for life- threatening tubal pregnancies.