Appendix Solutions of Exercises and Problems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

Compton Scattering from the Deuteron at Low Energies
SE0200264 LUNFD6-NFFR-1018 Compton Scattering from the Deuteron at Low Energies Magnus Lundin Department of Physics Lund University 2002 W' •sii" Compton spridning från deuteronen vid låga energier (populärvetenskaplig sammanfattning på svenska) Vid Compton spridning sprids fotonen elastiskt, dvs. utan att förlora energi, mot en annan partikel eller kärna. Kärnorna som användes i detta försök består av en proton och en neutron (sk. deuterium, eller tungt väte). Kärnorna bestrålades med fotoner av kända energier och de spridda fo- tonerna detekterades mha. stora Nal-detektorer som var placerade i olika vinklar runt strålmålet. Försöket utfördes under 8 veckor och genom att räkna antalet fotoner som kärnorna bestålades med och antalet spridda fo- toner i de olika detektorerna, kan sannolikheten för att en foton skall spridas bestämmas. Denna sannolikhet jämfördes med en teoretisk modell som beskriver sannolikheten för att en foton skall spridas elastiskt mot en deuterium- kärna. Eftersom protonen och neutronen består av kvarkar, vilka har en elektrisk laddning, kommer dessa att sträckas ut då de utsätts för ett elek- triskt fält (fotonen), dvs. de polariseras. Värdet (sannolikheten) som den teoretiska modellen ger, beror på polariserbarheten hos protonen och neu- tronen i deuterium. Genom att beräkna sannolikheten för fotonspridning för olika värden av polariserbarheterna, kan man se vilket värde som ger bäst överensstämmelse mellan modellen och experimentella data. Det är speciellt neutronens polariserbarhet som är av intresse, och denna kunde bestämmas i detta arbete. Organization Document name LUND UNIVERSITY DOCTORAL DISSERTATION Department of Physics Date of issue 2002.04.29 Division of Nuclear Physics Box 118 Sponsoring organization SE-22100 Lund Sweden Author (s) Magnus Lundin Title and subtitle Compton Scattering from the Deuteron at Low Energies Abstract A series of three Compton scattering experiments on deuterium have been performed at the high-resolution tagged-photon facility MAX-lab located in Lund, Sweden. -

The African School of Physics
The African School of Physics Lecture : Particle Interactions with Matter Version 2012 ASP2012 - SH Connell 1 Learning Goals, Material 1. Understand the fundamental interactions of high energy particles with matter. 1. High Energy Physics : 1. Understand the HEP detector design and operation. 2. Research in HEP 2. Nuclear Physics 1. Understand detector / shielding design and operation. 3. Medical Physics 1. Understand biological implications 2. Understand radiation therapy 4. Other 1. Environmental radiation 2. Radiation damage for Space applications 3. Semiconductor processing 4. Radiation Damage in Materials 2. The core material is from “Techniques for Nuclear and Particle Physics Experiments” by WR Leo. Supplementary material from ASP2010 and ASP2012 lecture notes. ASP2012 - SH Connell 2 Contents 1. Overview : Energy Loss mechanisms 2. Overview : Reaction Cross section and the probability of an interaction per unit path-length 3. Energy Loss mechanisms. 1. Heavy charged particles 2. Light charged particles 3. Photons 4. (Neutrons) 4. Multiple Coulomb Scattering 5. Energy loss distributions 6. Range of particles. 7. Radiation length 8. Showers 9. Counting Statistics ASP2012 - SH Connell 3 An example from the ATLAS detector Reconstruction of a 2e2μ candidate for the Higgs boson - with m2e2μ= 123.9 GeV We need to understand the interaction of particles with matter in order to understand the design and operation of this detector, and the analysis of the data. ASP2012 - SH Connell Energy Loss Mechanisms Heavy Charged Particles Light Charged -

Beta Decay Introduction
Beta decay Introduction Beta decay is the decay mechanism that affects the largest number of nuclei. In standard beta decay, or more specifically, beta-minus decay, a nucleus converts a neutron into a proton. The number of neutrons N decreases by one unit, and the number of protons Z increases by one. So the neutron excess decreases by two. Beta decay moves nuclei with too many neutrons closer to the stable range. Unlike the neutron, the proton has a positive charge, so by itself, converting a neutron into a proton would create charge out of nothing. However, that is not possible as net charge is preserved in nature. In beta decay, the nucleus also emits a negatively charged electron, making the net charge that is cre- ated zero as it should. But there is another problem with that. Now a neutron with spin 1/2 is converted into a proton and an electron, each with spin 1/2. That violates angular momentum conservation. (Regardless of any orbital angular momentum, the net angular momentum would change from half-integer to integer.) In beta de- cay, the nucleus also emits a second particle of spin 1/2, thus keeping the net angular momentum half- integer. Fermi called that second particle the neutrino, since it was electrically neutral and so small that it was initially impossible to observe. In fact, even at the time of writing, almost a century later, the mass of the neutrino, though known to be nonzero, is too small to measure. Nowadays the neutrino emitted in beta decay is more accurately identified as the electron antineutrino. -

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -

Interaction of Radiation with Matter
INTERACTION OF RADIATION WITH MATTER OBJECTIVES Aims From this chapter you should develop your understanding of the various ways that photons, charged particles and neutrons can interact with matter and the concepts, such as mass attenuation coefficient, stopping power and range, that have been invented in order to aid that understanding. These ideas are the basis for the later study of the effects of x rays, gamma radiation and other ionising radiations on living things. Minimum learning goals When you have finished studying this chapter you should be able to do all of the following. 1. Explain, use and interpret the terms linear attenuation coefficient, mass attenuation coefficient, attenuation length, mean free path, half thickness, density-thickness, build-up, secondary particles, photoelectric effect, atomic photoelectric effect, photoelectrons, photoionisation, absorption edges, Compton effect [Compton scattering], Compton edge, pair production, rate of energy loss, linear stopping power, mass stopping power, minimum ionisation, range, straggling, bremsstrahlung [braking radiation], Cherenkov radiation, elastic scattering. 2. State, explain and apply the exponential attenuation law for a beam of particles suffering all-or- nothing interactions. 3. State, explain and apply the relations among linear attenuation coefficient, mass attenuation coefficient, density and composition of materials. 4. Distinguish between absorption of energy and the attenuation of a beam of photons and describe the build-up of secondary particles. 5. (a) Describe and compare the processes (photoelectric effect, Compton effect, Rayleigh scattering, pair production) by which photons interact with matter. (b) Describe and explain in general terms how attenuation coefficients and the relative importance of those processes vary with photon energy and explain the origin of absorption edges. -
The Equation of Radiative Transfer How Does the Intensity of Radiation Change in the Presence of Emission and / Or Absorption?
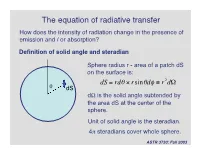
The equation of radiative transfer How does the intensity of radiation change in the presence of emission and / or absorption? Definition of solid angle and steradian Sphere radius r - area of a patch dS on the surface is: dS = rdq ¥ rsinqdf ≡ r2dW q dS dW is the solid angle subtended by the area dS at the center of the † sphere. Unit of solid angle is the steradian. 4p steradians cover whole sphere. ASTR 3730: Fall 2003 Definition of the specific intensity Construct an area dA normal to a light ray, and consider all the rays that pass through dA whose directions lie within a small solid angle dW. Solid angle dW dA The amount of energy passing through dA and into dW in time dt in frequency range dn is: dE = In dAdtdndW Specific intensity of the radiation. † ASTR 3730: Fall 2003 Compare with definition of the flux: specific intensity is very similar except it depends upon direction and frequency as well as location. Units of specific intensity are: erg s-1 cm-2 Hz-1 steradian-1 Same as Fn Another, more intuitive name for the specific intensity is brightness. ASTR 3730: Fall 2003 Simple relation between the flux and the specific intensity: Consider a small area dA, with light rays passing through it at all angles to the normal to the surface n: n o In If q = 90 , then light rays in that direction contribute zero net flux through area dA. q For rays at angle q, foreshortening reduces the effective area by a factor of cos(q). -

Neutrino Mysteries OLLI UC Irvine April 7, 2014
Neutrino Mysteries OLLI UC Irvine April 7, 2014 Dennis Silverman Department of Physics and Astronomy UC Irvine Neutrinos Around the Universe • Neutrinos • The Standard Model • The Weak Interactions Neutrino Oscillations • Solar Neutrinos • Atmospheric Neutrinos • Neutrino Masses • Neutrino vs. Antineutrino • Supernova Neutrinos Introduction to the Standard Model www.particleadventure.org Over 100 Years of Subatomic Physics Atoms to Electrons and Nuclei to Protons and Neutrons and to Quarks The size of a proton is about 10⁻¹³ cm, called a fermi. Protons have two up quarks and one down quark. Neutrons have one up quark and two down quarks. The Standard Model of Quarks and Leptons Electromagnetic, Weak, and Strong Color Interactions Q = +2/3 e Q = -1/3 e Q = 0 Q = - e The Spin of Particles, Charges, and Anti-particles • The quarks and leptons all have an intrinsic spin of ½ in units of hbar = h/2휋 =ħ, a very small number. These are called fermions after Enrico Fermi. They have anti-particles with opposite charges. • The up quarks have charge +2/3 of that of the electron’s magnitude, and the bottom quarks have charge -1/3. • The force particles have spin 1 times ħ, and are called bosons after S. N. Bose. • The force particles are their own antiparticles like Z⁰ and the photon, or in opposite pairs, like W⁺ and W⁻, and the colored gluons. Masses of Elementary Particles 125 GeV → The Proton and Neutron are about 1 GeV → A GeV is a giga electron volts in energy, or a billion electron volts Diagram from Gordon Kane, Scientific American 2003 The Weak Interactions The Beta (electron) Decay of a neutron is really that of a down quark to an up quark with a virtual W⁻ creating an electron and an electron anti-neutrino. -

Phase Transitions and the Casimir Effect in Neutron Stars
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 12-2017 Phase Transitions and the Casimir Effect in Neutron Stars William Patrick Moffitt University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Other Physics Commons Recommended Citation Moffitt, William Patrick, "Phase Transitions and the Casimir Effect in Neutron Stars. " Master's Thesis, University of Tennessee, 2017. https://trace.tennessee.edu/utk_gradthes/4956 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by William Patrick Moffitt entitled "Phaser T ansitions and the Casimir Effect in Neutron Stars." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Physics. Andrew W. Steiner, Major Professor We have read this thesis and recommend its acceptance: Marianne Breinig, Steve Johnston Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Phase Transitions and the Casimir Effect in Neutron Stars A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville William Patrick Moffitt December 2017 Abstract What lies at the core of a neutron star is still a highly debated topic, with both the composition and the physical interactions in question. -

Particle Production at Small-X in Deep Inelastic Scattering Dajing Wu Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2014 Particle production at small-x in deep inelastic scattering Dajing Wu Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Physics Commons Recommended Citation Wu, Dajing, "Particle production at small-x in deep inelastic scattering" (2014). Graduate Theses and Dissertations. 13846. https://lib.dr.iastate.edu/etd/13846 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Particle production at small-x in deep inelastic scattering by Dajing Wu A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Nuclear Physics Program of Study Committee: Kirill Tuchin, Major Professor James Vary Marzia Rosati Kerry Whisnant Alexander Roitershtein Iowa State University Ames, Iowa 2014 Copyright © Dajing Wu, 2014. All rights reserved. ii DEDICATION I would like to dedicate this thesis to my father, Xianggui Wu, and my mother, Wenfang Tang. Without their encouragement and support, I could not have completed such work. iii TABLE OF CONTENTS LIST OF FIGURES . vi ABSTRACT . x ACKNOWLEGEMENT . xi PART I Theoretical foundations for small-x physics1 CHAPTER 1. QCD as a theory for strong interactions . 2 1.1 QCD Lagrangian . .2 1.2 Perturbative QCD . .2 1.3 Light-cone perturbation theory . -

Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit .