The Biogeography of Lungfishes with a Description of New Fossil Taxa from East Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

This Content Downloaded from 157.193.10.229 on Tue, 07 Jul
This content downloaded from 157.193.10.229 on Tue, 07 Jul 2015 14:17:10 UTC All use subject to JSTOR Terms and Conditions CLEMENT and BOISVERT-DEVONIAN LUNGFISH FROM BELGIUM 277 tra. In addition to his incorrect taxonomic attribution, Lohest idae Berg, 1940 (including Fleurantia and Jarvikia); and Rhyn- misinterpreted the operculum as a scapula, the cleithrum as a chodipteridae Moy-Thomas, 1939 (including Rhynchodipterus, coracoid, and the E bone as an isolated rib (Fig. 2A, B). How- Griphognathus, and Soederberghia). Schultze (1993) defined the ever, he accurately identified a pleural rib (Fig. 2A, B). Rhynchodipteridae as including at least Soederberghia, Jarvikia, and Fleurantia. Later, Schultze (2001) presented a cladogram of SYSTEMATIC PALEONTOLOGY Devonian dipnoans that included a radiation of denticulated forms: Barwickia [Fleurantia + Rhynchodipteridae], in which included SARCOPTERYGII Romer, 1955 Rhynchodipteridae Griphognathus [Rhynchodipterus + The and affinities of the DIPNOMORPHA Ahlberg, 1991 [Soederberghia Jarvikia]]. monophyly DIPNOI 1845 Rhynchodipteridae have been reviewed by Ahlberg et al. (2001), Muiller, who that be unrelated RHYNCHODIPTERIDAE Moy-Thomas, 1939 tentatively suggested Griphognathus may to Rhynchodipterus and Soederberghia, but regarded Rhyncho- Remarks-Campbell and Barwick (1990) proposed that the dipterus and Soederberghia as most closely related to each other. denticulated lungfish lineage should be recognized as suborder However, Friedman (2003b) considered this suggestion prema- Uranolophina which incorporates four families: Uranolophidae ture and suggested that the Rhynchodipteridae, if defined as Miles, 1977; Holodontidae Gorizdro-Kulczycka, 1950; Fleuranti- including only Soederberghia, Rhynchodipterus, and Griphogna- FIGURE 2. Soederberghiasp. indet. Modave, Liege Province, Belgium, upper Famennian,Upper Devonian. Liege University, paleontology collection no. 5390a,b. A, no. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Cambridge University Press 978-1-107-17944-8 — Evolution And
Cambridge University Press 978-1-107-17944-8 — Evolution and Development of Fishes Edited by Zerina Johanson , Charlie Underwood , Martha Richter Index More Information Index abaxial muscle,33 Alizarin red, 110 arandaspids, 5, 61–62 abdominal muscles, 212 Alizarin red S whole mount staining, 127 Arandaspis, 5, 61, 69, 147 ability to repair fractures, 129 Allenypterus, 253 arcocentra, 192 Acanthodes, 14, 79, 83, 89–90, 104, 105–107, allometric growth, 129 Arctic char, 130 123, 152, 152, 156, 213, 221, 226 alveolar bone, 134 arcualia, 4, 49, 115, 146, 191, 206 Acanthodians, 3, 7, 13–15, 18, 23, 29, 63–65, Alx, 36, 47 areolar calcification, 114 68–69, 75, 79, 82, 84, 87–89, 91, 99, 102, Amdeh Formation, 61 areolar cartilage, 192 104–106, 114, 123, 148–149, 152–153, ameloblasts, 134 areolar mineralisation, 113 156, 160, 189, 192, 195, 198–199, 207, Amia, 154, 185, 190, 193, 258 Areyongalepis,7,64–65 213, 217–218, 220 ammocoete, 30, 40, 51, 56–57, 176, 206, 208, Argentina, 60–61, 67 Acanthodiformes, 14, 68 218 armoured agnathans, 150 Acanthodii, 152 amphiaspids, 5, 27 Arthrodira, 12, 24, 26, 28, 74, 82–84, 86, 194, Acanthomorpha, 20 amphibians, 1, 20, 150, 172, 180–182, 245, 248, 209, 222 Acanthostega, 22, 155–156, 255–258, 260 255–256 arthrodires, 7, 11–13, 22, 28, 71–72, 74–75, Acanthothoraci, 24, 74, 83 amphioxus, 49, 54–55, 124, 145, 155, 157, 159, 80–84, 152, 192, 207, 209, 212–213, 215, Acanthothoracida, 11 206, 224, 243–244, 249–250 219–220 acanthothoracids, 7, 12, 74, 81–82, 211, 215, Amphioxus, 120 Ascl,36 219 Amphystylic, 148 Asiaceratodus,21 -

Identifying Heterogeneity in Rates of Morphological Evolution: Discrete Character Change in the Evolution of Lungfish (Sarcopterygii; Dipnoi)
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2011.01460.x IDENTIFYING HETEROGENEITY IN RATES OF MORPHOLOGICAL EVOLUTION: DISCRETE CHARACTER CHANGE IN THE EVOLUTION OF LUNGFISH (SARCOPTERYGII; DIPNOI) Graeme T. Lloyd,1,2 Steve C. Wang,3 and Stephen L. Brusatte4,5 1Department of Palaeontology, Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom 2E-mail: [email protected] 3Department of Mathematics and Statistics, Swarthmore College, Swarthmore, Pennsylvania 19081 4Division of Paleontology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024 5Department of Earth and Environmental Sciences, Columbia University, New York, New York 10025 Received February 9, 2010 Accepted August 15, 2011 Data Archived: Dryad: doi:10.5061/dryad.pg46f Quantifying rates of morphological evolution is important in many macroevolutionary studies, and critical when assessing possible adaptive radiations and episodes of punctuated equilibrium in the fossil record. However, studies of morphological rates of change have lagged behind those on taxonomic diversification, and most authors have focused on continuous characters and quantifying patterns of morphological rates over time. Here, we provide a phylogenetic approach, using discrete characters and three statistical tests to determine points on a cladogram (branches or entire clades) that are characterized by significantly high or low rates of change. These methods include a randomization approach that identifies branches with significantly high rates and likelihood ratio tests that pinpoint either branches or clades that have significantly higher or lower rates than the pooled rate of the remainder of the tree. As a test case for these methods, we analyze a discrete character dataset of lungfish, which have long been regarded as “living fossils” due to an apparent slowdown in rates since the Devonian. -

Blackchin Tilapia (Sarotherodon Melanotheron) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Blackchin Tilapia (Sarotherodon melanotheron) Ecological Risk Screening Summary Web Version – 10/01/2012 Photo: © U.S. Geological Survey From Nico and Neilson (2014). 1 Native Range and Nonindigenous Occurrences Native Range From Nico and Neilson (2014): “Tropical Africa. Brackish estuaries and lagoons from Senegal to Zaire (Trewavas 1983).” Nonindigenous Occurrences From Nico and Neilson (2014): “Established in Florida and Hawaii. Evidence indicates it is spreading rapidly in both fresh and salt water around island of Oahu, Hawaii (Devick 1991b).” “The first documented occurrence of this species in Florida was a specimen gillnetted by commercial fishermen in Hillsborough Bay near Tampa, Hillsborough County, in 1959 (Springer and Finucane 1963). Additional records for the western part of the state indicate that this species is established in brackish and freshwaters in eastern Tampa Bay and in adjoining drainages in Hillsborough County, ranging from the Alafia River south to Cockroach Bay. The species has been recorded from the Alafia River from its mouth up to Lithia Springs; from the Hillsborough River, Bullfrog Creek, the Palm River, and the Little Manatee River; and from various western drainage and irrigation ditches (Springer and Finucane 1963; Finucane and Rinckey 1967; Buntz Sarotherodon melanotheron Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 10/01/2012 and Manooch 1969; Lachner et al. 1970; Courtenay et al. 1974; Courtenay and Hensley 1979; Courtenay and Kohler 1986; Lee et al. 1980 et seq.; Courtenay and Stauffer 1990; DNR collections; UF museum specimens). There are two records of this species from the west side of Tampa Bay, in Pinellas County: a collection from Lake Maggiore in St. -

New Locality for Lepidosiren Paradoxa (Fitzinger, 1837)(Dipnoi
13 3 the journal of 2118 biodiversity data 20 May 2017 Check List NOTES ON GEOGRAPHIC DISTRIBUTION Check List 13(3): 2118, 20 May 2017 https://doi.org/10.15560/13.3.2118 ISSN 1809-127X © 2017 Check List and Authors New locality for Lepidosiren paradoxa (Fitzinger, 1837) (Dipnoi: Lepidosirenidae) in Argentina Evelyn Romina Vallone Laboratorio de Paleontología de Vertebrados, Centro de Investigaciones Científicas y Transferencia de Tecnología a la Producción (CICyTTP-CONICET), Materi y España, 3105 Diamante, ER, Argentina E-mail: [email protected] Abstract. This study documents a new locality of the lungfish was caught by a local fisherman in shallow waters (depth of Lepidosiren paradoxa on the coast of the Entre Rios Province approximately 3 m) in a coastal area of the locality of Gen- in the lower Paraná River. This finding represents the third eral Alvear, Entre Ríos Province, Argentina (31°55ʹ27.60ʺ S, southern record of this species on southern of South America. 060°39ʹ45.52ʺ W) (Fig. 1). After collection, the specimen was Additionally, as this region has been relatively well sampled frozen and transported to the laboratory for identification. The both during past decades and currently, I discuss possible specimen was identified according to diagnosis of Ringuelet reasons why this new specimen has been observed only et al. (1967) then deposited in the Fish Collection of the Labo- recently. ratorio de Vertebrados, Centro de Investigaciones Científicas y Key words. South American lungfish; Entre Ríos; Paraná River Transferencia de Tecnología a la Producción (CICyTTP-V-18, Diamante, Entre Ríos, Argentina). The description follows the anatomical nomenclature proposed by Ringuelet et al. -

Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha)
Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha) by Richard Kissel A thesis submitted in conformity with the requirements for the degree of doctor of philosophy Graduate Department of Ecology & Evolutionary Biology University of Toronto © Copyright by Richard Kissel 2010 Morphology, Phylogeny, and Evolution of Diadectidae (Cotylosauria: Diadectomorpha) Richard Kissel Doctor of Philosophy Graduate Department of Ecology & Evolutionary Biology University of Toronto 2010 Abstract Based on dental, cranial, and postcranial anatomy, members of the Permo-Carboniferous clade Diadectidae are generally regarded as the earliest tetrapods capable of processing high-fiber plant material; presented here is a review of diadectid morphology, phylogeny, taxonomy, and paleozoogeography. Phylogenetic analyses support the monophyly of Diadectidae within Diadectomorpha, the sister-group to Amniota, with Limnoscelis as the sister-taxon to Tseajaia + Diadectidae. Analysis of diadectid interrelationships of all known taxa for which adequate specimens and information are known—the first of its kind conducted—positions Ambedus pusillus as the sister-taxon to all other forms, with Diadectes sanmiguelensis, Orobates pabsti, Desmatodon hesperis, Diadectes absitus, and (Diadectes sideropelicus + Diadectes tenuitectes + Diasparactus zenos) representing progressively more derived taxa in a series of nested clades. In light of these results, it is recommended herein that the species Diadectes sanmiguelensis be referred to the new genus -

Geological Survey of Ohio
GEOLOGICAL SURVEY OF OHIO. VOL. I.—PART II. PALÆONTOLOGY. SECTION II. DESCRIPTIONS OF FOSSIL FISHES. BY J. S. NEWBERRY. Digital version copyrighted ©2012 by Don Chesnut. THE CLASSIFICATION AND GEOLOGICAL DISTRIBUTION OF OUR FOSSIL FISHES. So little is generally known in regard to American fossil fishes, that I have thought the notes which I now give upon some of them would be more interesting and intelligible if those into whose hands they will fall could have a more comprehensive view of this branch of palæontology than they afford. I shall therefore preface the descriptions which follow with a few words on the geological distribution of our Palæozoic fishes, and on the relations which they sustain to fossil forms found in other countries, and to living fishes. This seems the more necessary, as no summary of what is known of our fossil fishes has ever been given, and the literature of the subject is so scattered through scientific journals and the proceedings of learned societies, as to be practically inaccessible to most of those who will be readers of this report. I. THE ZOOLOGICAL RELATIONS OF OUR FOSSIL FISHES. To the common observer, the class of Fishes seems to be well defined and quite distin ct from all the other groups o f vertebrate animals; but the comparative anatomist finds in certain unusual and aberrant forms peculiarities of structure which link the Fishes to the Invertebrates below and Amphibians above, in such a way as to render it difficult, if not impossible, to draw the lines sharply between these great groups. -

Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships
438 Article 438 by Saswati Bandyopadhyay1* and Sanghamitra Ray2 Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships 1Geological Studies Unit, Indian Statistical Institute, 203 B. T. Road, Kolkata 700108, India; email: [email protected] 2Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur 721302, India; email: [email protected] *Corresponding author (Received : 23/12/2018; Revised accepted : 11/09/2019) https://doi.org/10.18814/epiiugs/2020/020028 The twelve Gondwanan stratigraphic horizons of many extant lineages, producing highly diverse terrestrial vertebrates India have yielded varied vertebrate fossils. The oldest in the vacant niches created throughout the world due to the end- Permian extinction event. Diapsids diversified rapidly by the Middle fossil record is the Endothiodon-dominated multitaxic Triassic in to many communities of continental tetrapods, whereas Kundaram fauna, which correlates the Kundaram the non-mammalian synapsids became a minor components for the Formation with several other coeval Late Permian remainder of the Mesozoic Era. The Gondwana basins of peninsular horizons of South Africa, Zambia, Tanzania, India (Fig. 1A) aptly exemplify the diverse vertebrate faunas found Mozambique, Malawi, Madagascar and Brazil. The from the Late Palaeozoic and Mesozoic. During the last few decades much emphasis was given on explorations and excavations of Permian-Triassic transition in India is marked by vertebrate fossils in these basins which have yielded many new fossil distinct taxonomic shift and faunal characteristics and vertebrates, significant both in numbers and diversity of genera, and represented by small-sized holdover fauna of the providing information on their taphonomy, taxonomy, phylogeny, Early Triassic Panchet and Kamthi fauna. -

Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone
Southampton French Quarter SOU1382 Specialist Report Download E2 Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone By Rebecca Nicholson Introduction An assemblage of almost 7500 identifiable fish bones was recovered both by hand retrieval during the excavation, but predominantly from the sorted residues of the processed bulk soil samples. During excavations at Southampton French Quarter a total of 188 bulk samples were sieved to 0.5mm (occasionally 1mm) as part of the flotation process for the recovery of plant and animal remains. The sampling strategy followed during the excavation involved, where possible, the full sampling of one rubbish pit and one latrine pit per tenement for each major period, avoiding intercut features or those clearly containing residual material. Occupation surfaces and other distinct features such as hearths were also sampled. Mixed contexts or contexts of uncertain provenance were avoided. While complete standardisation of sample volumes was not possible, wherever practicable samples were 40 litres. Following assessment of the fish remains recovered largely from the > 4mm residues, the richer assemblages were targeted for further fine residue and flot sorting. This report comprises an analysis of all the identified the fish remains from these samples, together with the material collected by hand on site. Methodology The residues from all of the bulk-sieved samples were sorted to 4mm. Where samples were identified as having significant numbers of fish bones, residues were sorted to 2mm. Samples from the Late Saxon deposits which produced fish remains were routinely sorted to 2mm even where fish remains were not abundant, in order to avoid a perceived bias against the recovery of small fish in pre-medieval deposits (see Barrett et al. -
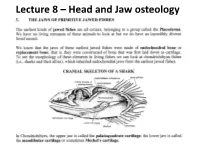
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

A Redescription of the Lungfish Eoctenodus Hills 1929, with Reassessment of Other Australian Records of the Genus Dipterns Sedgwick & Murchison 1828
Ree. West. Aust. Mus. 1987, 13 (2): 297-314 A redescription of the lungfish Eoctenodus Hills 1929, with reassessment of other Australian records of the genus Dipterns Sedgwick & Murchison 1828. J.A. Long* Abstract Eoctenodus microsoma Hills 1929 (= Dipterus microsoma Hills, 1931) from the Frasnian Blue Range Formation, near Taggerty, Victoria, is found to be a valid genus, differing from Dipterus, and other dipnoans, by the shape of the parasphenoid and toothplates. The upper jaw toothp1ates and entopterygoids, parasphenoid, c1eithrum, anoc1eithrum and scales of Eoctenodus are described. Eoctenodus may represent the earliest member of the Ctenodontidae. Dipterus cf. D. digitatus. from the Late Devonian Gneudna Formation, Western Australia (Seddon, 1969), is assigned to Chirodipterus australis Miles 1977; and Dipterus sp. from the Late Devonian of Gingham Gap, New South Wales (Hills, 1936) is thought to be con generic with a dipnoan of similar age from the Hunter Siltstone, New South Wales. This form differs from Dipterus in the shape of the parasphenoid. The genus Dipterus appears to be restricted to the Middle-Upper Devonian of Europe, North America and the USSR (Laurasia). Introduction Although Hills (1929) recognised a new dipnoan, Eoctenodus microsoma, in the Late Devonian fish remains from the Blue Range Formation, near Taggerty, he later (Hills 1931) altered the generic status of this species after a study trip to Britain in which D,M.S. Watson pointed out similarities between the Australian form and the British genus Dipterus Sedgwick and Murchison 1828. Studies of the head of Dipterus by Westoll (1949) and White (1965) showed the structure of the palate and, in particular, the shape of the parasphenoid which differs from that in the Taggerty dipnoan.